Recent research has unveiled crucial insights into the regulation of phosphate transport in human cells, shedding light on the functions of the XPR1 protein and its regulatory relationship with inositol pyrophosphate (InsP8). This discovery is poised to enhance our understanding of phosphate's vital role in cellular metabolism and its impact on health.
Phosphate is essential for various cellular processes, including energy production and genetic material formation. The intracellular concentration of inorganic phosphate (Pi) must be tightly controlled, and the XPR1 exporter plays a pivotal role in maintaining this balance. Abnormalities in XPR1 function are linked to serious neurological disorders and cancers, highlighting the need for a clear understanding of its mechanisms.
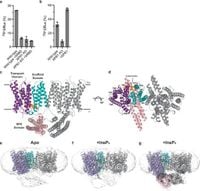
In this groundbreaking study, authors conducted a series of cryo-electron microscopy analyses revealing the structure of XPR1 in different conformational states. They established that when InsP8 binds to XPR1, it locks the SPX domain in a fixed position, which is essential for activating phosphate transport. The researchers noted, “Binding of InsP8 to XPR1... rigidifies the intracellular SPX domains, with InsP8 bridging the dimers and SPX and transmembrane domains.” This structural insight elucidates how a single molecule—InsP8—modulates the functionality of XPR1, emphasizing its role as a key regulator of phosphate efflux.
The study highlights the various states of the XPR1 transporter and the significance of the dynamics of the SPX domain in the transport process. In their findings, the authors demonstrated that InsP8 is the only physiologically relevant ligand that facilitates the transport process through XPR1, surpassing other inositol polyphosphates like InsP6.
As the research demonstrates, XPR1 dimerization is vital for its function. This dimeric form allows the transporter to effectively transport Pi out of the cell while also indicating its structural integrity is essential for maintaining its activity. Out-of-balance phosphate levels due to XPR1 mutation can lead to brain calcifications, which are associated with neurological disorders such as movement disorders and psychosis.
Moreover, the implications of this research extend into the realm of oncology. Elevated phosphate requirements are found in proliferative cells, typifying certain cancers. The overactivation of phosphate importers is a common feature in cancer, particularly in ovarian and uterine tumors. The authors pointed towards the potential for targeting XPR1 with inhibitors as a form of treatment, proposing that “inhibition of XPR1 is an attractive treatment option for these highly lethal cancers.” This therapeutic angle presents a dual benefit; modulation of XPR1 could prevent excessive phosphate retention in cancer cells while managing associated neurological disorders linked to XPR1 dysfunction.
Additionally, the study enhances the understanding of how phosphate homeostasis is managed at a cellular level, revealing how precise mechanisms can dictate broader physiological responses. The importance of this regulation is further highlighted by the authors’ assertion that “these findings advance our understanding of XPR1 transport activity and expand opportunities for rationalizing disease mechanisms and therapeutic intervention.”
In light of these revelations, the work showcases major therapeutic avenues that could be pursued for conditions involving disrupted phosphate balance. As structural biology techniques continue to progress, future research may unearth even more nuanced relationships between XPR1, InsP8, and cell signaling pathways, ultimately facilitating new treatment strategies for patients affected by XPR1-related diseases.
The quest to fully decipher the complexities of phosphate transport remains ongoing. As scientists continue to explore these pathways, the integration of such molecular findings may equip clinicians with better tools for intervention in various disease states affected by phosphate dysregulation.