In an innovative study exploring the intricacies of locomotor control in mammals, researchers have uncovered the distinct functions of two subtypes of striatonigral neurons, Kremen1+ and Calb1+, in regulating movement. Traditionally recognized for their role in facilitating locomotion, these neurons exhibit opposing characteristics that may transform our understanding of how movement is controlled in the brain.
The investigation, conducted using mouse models, highlights that Kremen1+ neurons activate later during movement onset and play a critical role in terminating locomotion, while Calb1+ neurons are crucial for effective movement initiation. This means that our previous understanding of striatonigral neurons may have been overly simplistic and that the striatum — a crucial part of the brain involved in motor control — consists of diverse neuron types that contribute uniquely to motor behavior.
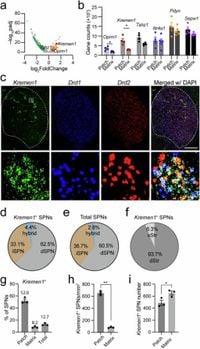
Through the use of genetic markers, Kremen1 and Calb1, the researchers are able to demonstrate how these neuron types function distinctly. Activation of Kremen1+ neurons suppresses ongoing locomotion, while the activation of Calb1+ neurons encourages movement. These findings point to a significant shift in how scientists may view basal ganglia circuitry, with implications for understanding disorders like Parkinson's disease, where motor function can be severely impaired.
The researchers utilized genetic modifications and advanced methodologies, including optogenetic stimulation, to uncover these mechanisms. For instance, upon activating Kremen1+ neurons, the study observed a decrease in dopamine release compared to Calb1+ neurons, which corresponded with locomotion suppression. Optogenetic experiments involved injecting channels that respond to light into specific neuron types, allowing researchers to activate or inhibit them selectively. This level of control provided crucial insights into the temporal dynamics of neuron activity during locomotion.
Interestingly, during self-paced locomotion, the Kremen1+ neurons exhibited a delay in their peak activity compared to Calb1+ neurons, suggesting a nuanced regulatory role that might facilitate the cessation of movement. In contrast, Calb1+ neurons showed increased activity prior to the start of movement — a clear indicator of their involvement in locomotor initiation.
Additionally, the study delved into the impact of these neuron types on dopamine release, a neurotransmitter known to play a pivotal role in reward and motor function. Activation of Kremen1+ neurons induced a significant drop in dopamine, linked to the suppression of locomotion, which was not observed to the same degree with the Calb1+ neurons. This suggests that the pathways governing locomotion are intricately tied to dopamine signaling, further complicating the landscape of motor control in the brain.
Furthermore, the tactical removal of GABA-B receptors in specific dopaminergic neurons eliminated the locomotion-suppressing effect of Kremen1+ neurons, illustrating the mechanistic pathways through which these neurons exert their influence over motor control. These insights could pave the way for more targeted interventions in motor disorders, especially as research continues to uncover the complexities of these neuronal interactions in states of health and disease.
Overall, the findings presented in this study underscore the importance of recognizing the diversity within the striatonigral neuron population and how distinct subpopulations can be harnessed for specific functions in locomotion. Such revelations not only advance our understanding of brain function related to movement but also herald potential new avenues for treating motor disorders by focusing on the distinct neuronal pathways involved in regulating locomotion. The researchers emphasize the need for a more refined approach to studying the basal ganglia, as their complex relationships and interactions may reveal further secrets about movement and its regulation in the mammalian brain.