A groundbreaking study has unveiled the molecular mechanism connecting cigarette smoking to the worsening of age-related macular degeneration (AMD), a leading cause of blindness among older adults. This research focuses on the role of the Sema4D-PlexinB1 signaling pathway that is activated in response to smoking, revealing how it exacerbates the progression of AMD and affects the efficacy of treatments.
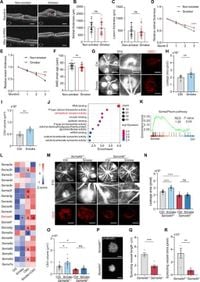
The study highlights that smoking significantly increases the levels of Sema4D, a protein expressed by CD8+ T cells, which migrate into lesions associated with choroidal neovascularization (CNV), a severe form of AMD. There, Sema4D interacts with PlexinB1 on pericytes, leading to their activation and malfunction, ultimately resulting in vascular instability.
Using patient samples and mouse models, the researchers demonstrated that targeting Sema4D could not only inhibit CNV progression but also enhance the effectiveness of anti-VEGF therapies, such as Conbercept. This dual approach may represent a promising new strategy for treating AMD, especially for patients who are smokers.
Age-related macular degeneration (AMD) is a neuroinflammatory disease recognized as the leading cause of irreversible vision loss in the elderly worldwide. The prevalence of AMD is increasing due to a growing aging population, making it a pressing public health concern. Established risk factors for AMD include advanced age and cigarette smoking, with studies indicating that smoking increases the incidence of neovascular AMD (nvAMD) and decreases the responsiveness to therapies.
Neovascular AMD is characterized by CNV, where abnormal blood vessels grow beneath the retina, causing vision loss. The introduction of anti-VEGF therapy has improved outcomes for some patients; however, consistently poor results are seen in a significant number of cases. This research shines a new light on the underlying mechanisms of AMD as affected by smoking.
Pericytes are critical for vascular health, providing stability to blood vessels. The study found that when smoking activates the Sema4D-PlexinB1 pathway, it leads to pericyte dysfunction, contributing to vascular leakage and the proliferation of aberrant blood vessels that typify AMD pathology. The interaction between CD8+ T cells and pericytes plays a significant role in this cascade of events.
The authors discovered that smoking exposure notably elevated the expression of Sema4D on CD8+ T cells, which were found to be present in greater numbers within the AMD lesions of smokers. These T cells utilize the CXCL12-CXCR4 signaling axis to migrate to these areas, where they exert detrimental effects through Sema4D-PlexinB1 interactions with pericytes.
In their experiments, the researchers were able to corroborate that inhibiting Sema4D led to reduced CNV progression in mouse models, along with improved outcomes from anti-VEGF treatments. Specifically, the combination of anti-Sema4D therapy and anti-VEGF therapy provided enhanced vascular stability and reduced visual impairment in treated models.
These findings emphasize the need for targeted therapies that consider the impact of smoking on AMD, focusing on the Sema4D-PlexinB1 signaling pathway as a viable target for development. As ongoing research continues to refine these therapeutic strategies, there is hope for improved outcomes in patients with smoking-related AMD.
The study presents an opportunity to rethink treatment approaches for AMD, particularly in smokers, and provides insights into the complex interplay between inflammation, vascular health, and neurodegenerative conditions affecting the retina.