New discoveries in the field of hematology underscore the intricate relationship between genomic elements and cellular metabolism in the development of red blood cells. A team of researchers has revealed that the gene Nprl3 plays a pivotal role in the process of erythropoiesis, which is essential for the production of red blood cells in the human body.
Erythropoiesis is a highly efficient process that produces approximately 2 million red blood cells per second, marking it as one of the most significant metabolic challenges in the human body. The regulation of this process requires fine-tuned transcriptional control of the α- and β-globin genes, and recent studies have placed Nprl3—a gene conserved across over 500 million years—at the center of this regulation.
Researchers discovered that while hematopoietic progenitors require a baseline level of Nprl3 expression, further studies indicated that its expression is significantly upregulated in erythroid cells by α-globin enhancers, which is crucial for sufficient erythropoiesis. Loss of Nprl3 not only disrupts this process but also leads to elevated signaling through the mTORC1 pathway. This dysregulation suppresses autophagy—an essential process for cellular health—and alters glycolysis, leading to inefficiencies in red blood cell production.
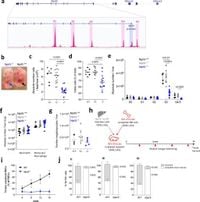
Through the use of knockout models and in vitro cell cultures, the study meticulously tracked how loss of Nprl3 affects both murine and human erythroid progenitors. Findings revealed that Nprl3-deficient erythroid progenitors in mice produced fewer enucleated cells and exhibited dysregulated mTORC1 signaling when responding to nutrient availability, as well as erythropoietin (EPO)—a hormone that stimulates the production of red blood cells.
In a detailed examination of both embryonic and adult blood cell production, it was evident that lack of Nprl3 led to severe impairments in erythropoiesis. The research showed that in models lacking Nprl3, there were significant reductions in the size and viability of fetal liver, leading to diminished erythroid cell populations. The failure in differentiation at crucial stages of erythropoiesis underscored the importance of Nprl3 as a metabolic regulator.
Further investigations into the mechanisms revealed that Nprl3 acts as a critical regulator of mTORC1, a central pathway involved in cellular growth and metabolism. As mTORC1 signals the anabolic processes necessary for hemoglobin synthesis while inhibiting catabolic pathways such as autophagy, its dysregulated activity due to Nprl3 loss illustrates how vital metabolic balance is to the maturation of red blood cells.
The findings also proposed an ancient genomic partnership where Nprl3, α-globin, and their corresponding enhancers not only facilitate the successful synthesis of hemoglobin but also ensure tight regulation of metabolic pathways that support erythroid cell creation. "We propose that the anciently conserved linkage of Nprl3, α-globin, and their associated enhancers has coupled metabolic and developmental control of erythropoiesis," wrote the authors of the article, highlighting the evolutionary significance of their work.
Moreover, the expression of Nprl3 was shown to increase with erythroid commitment. The hormonal and nutritional stimuli impacting CD34+ hematopoietic stem and progenitor cells (HSPCs) were also tested, indicating that NPRL3 is required for the proper integration of various signals influencing erythropoiesis. Controlled experiments demonstrated that Nprl3-deficient erythroid progenitors failed to maintain expected mTORC1 activity in response to essential nutrients and EPO, leading to profound implications for understanding both normal physiological processes and blood disorders.
In the study’s conclusion, the authors emphasized the dual role of Nprl3 not just in erythropoiesis but also as a multifaceted player in the regulation of metabolism. The researchers suggested that the loss of Nprl3 impacts autophagy and metabolic homeostasis, contributing to developmental failures in cell differentiation that could inform future studies on blood-related diseases.
These promising insights connect metabolic regulation with genetic control in red blood cell development, paving the way for potential therapeutic approaches for various hematological disorders where erythropoiesis is impaired. By defining the specific contributions of Nprl3 and its enhancer interactions, researchers hope to deepen the understanding of how red blood cells adapt to metabolic demands, ultimately enhancing strategies for clinical interventions in anemia and other blood disorders.