Researchers have identified a new mechanism contributing to the self-assembly of molecular structures in solutions, utilizing an effect known as the electrosolvation force. This discovery enhances the understanding of how small changes in solvent composition can dramatically influence molecular interactions and assembly processes relevant to numerous fields, including drug delivery and biomolecular stability.
The process of self-assembly is fundamental in many natural and industrial systems, guiding the behavior of everything from proteins folding in our bodies to the crystallization of materials. In most cases, this phenomenon arises from attractive molecular interactions that help to overcome the disorder introduced by entropy. However, recent studies have shed light on the electrosolvation force, offering more insight into how matter organizes at a molecular level.
According to the study led by a team of scientists from various institutions, the electrosolvation force is linked to the structure of the interfacial electrolyte where molecules interact. This discovery shows that neutral molecules, including different solvents, osmolytes, and surfactants, can disrupt or reinforce the existing interfacial solvent structure, offering an unprecedented way to tune the interactions that drive self-assembly.
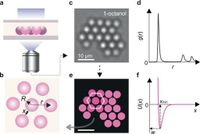
The researchers explored these effects through a series of innovative experiments, examining how suspensions of colloidal particles behave in various solvents, including water and alcohols of varying chain length. The results revealed that even minimal changes in the solution can lead to significant alterations in how particles cluster and form structures.
To further articulate the findings, the study detailed how the structure of liquid at the particle interface affects the overall system's behavior. “With the electrosolvation force, we see that the interactions between similar charged particles can actually change from repulsive to attractive based on the solvent’s properties,” stated the authors of the article. The interactions observed challenge traditional notions of molecular behavior, showing how counterintuitive behaviors can arise in charged particle systems under specific solvent conditions.
For instance, negatively charged silica particles were found to attract each other at long range in certain solvents while positively charged particles experienced repulsion. This insight could lead to developments that improve the stability of colloids and enhance the efficiency of processes that rely on these interactions, such as environmental remediation or the formulation of nanomaterials.
The comprehensive analysis employed advanced methodologies, including bright field microscopy and molecular dynamics simulations, allowing researchers to visualize and quantify the interactions between particles in a solution. By combining experimental data with theoretical modeling, the scientists could effectively validate the existence and role of the electrosolvation force in multiple solvents.
As researchers further elucidate intricate interactions at the microscopic level, these findings could open new doors in fields such as biochemistry, materials science, and nanotechnology. The ability to control assembly and stability through chemical tuning of solvents presents exciting possibilities for innovation.
Conclusively, the novel insights into the electrosolvation force not only enhance the current understanding of molecular interactions but may also contribute efficacious strategies in applications ranging from drug formulation to the development of advanced materials. Future studies investigating these dynamics, especially within complex biological environments, will likely be essential for leveraging these findings in practical applications.