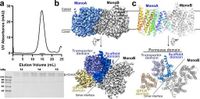
In groundbreaking research published in Nature Communications, scientists have unveiled the intricate structure of the lysosomal membrane protein known as LYCHOS, revealing vital insights into its role as a cholesterol sensor within human cells. Utilizing advanced cryo-electron microscopy, the team successfully determined the structure of LYCHOS at an impressive resolution of 3.1 Å, identifying a cholesterol-like density located at the interface between its permease and G-protein coupled receptor (GPCR) domains.
This research highlights the essential function of LYCHOS in regulating mTORC1, a critical nutrient-sensing pathway that coordinates cellular responses to nutrient availability, particularly cholesterol. The study elucidates how LYCHOS connects the concentration of lysosomal cholesterol to the activation of mTORC1, thereby facilitating key biosynthetic processes necessary for cell proliferation.
Researchers focused on analyzing the cryo-EM data, collecting a total of 4228 micrographs of LYCHOS during this investigation. This extensive data set led to the identification of significant conformational changes in the GPCR domain relative to the permease domain, a key discovery that enhances our understanding of how LYCHOS senses varying cholesterol levels.
In addition to its cholesterol-sensing capabilities, the study identified a fascinating interaction between LYCHOS and indoxyl sulfate (IS), a gut-derived uremic toxin implicated in chronic kidney diseases. The binding affinity of LYCHOS to IS was measured with a dissociation constant (Kd) of approximately 150 µM, indicating that this interaction has potential implications for metabolic regulation within lysosomes.
"This research provides structural insights into how LYCHOS senses lysosomal cholesterol levels and activates the mTORC1 signaling pathway," stated the authors of the article. Their findings underscore the importance of maintaining cholesterol homeostasis to prevent conditions such as atherosclerosis, obesity, and neurodegenerative diseases.
Crucially, the study demonstrates that the permease domain of LYCHOS possesses the capability to bind IS, which was confirmed through the structural analysis of the LYCHOS-IS complex at a resolution of 3.4 Å. This binding interaction may potentially influence how LYCHOS regulates mTORC1 activity, especially in the context of chronic kidney disease.
The intricate design of LYCHOS includes a total of 17 transmembrane segments divided between the N-terminal permease domain, which facilitates substrate transport, and the C-terminal GPCR domain involved in signaling pathways. The overall configuration of these domains suggests that LYCHOS operates with a unique structure that aligns it closely with known transporters and GPCRs.
Among the critical findings, the research also emphasizes the role of two lipids, termed Lipid1 and Lipid2, nestled between the permease and GPCR domains. These lipids are posited to stabilize the interaction between these two functional domains, essential for the effective operation of LYCHOS.
The study opens avenues for further questions regarding the specific mechanisms by which LYCHOS modulates cholesterol-induced activation of mTORC1 and its possible implications in disease states. As researchers continue to investigate the pathways involved in cholesterol metabolism and regulation, the insights gained from this structural analysis will undoubtedly pave the way for novel therapies targeting related metabolic disorders.
In conclusion, the research published in Nature Communications not only enhances our structural understanding of LYCHOS but also highlights its integral role in cellular cholesterol sensing and metabolism. Future investigations could lead to critical breakthroughs in managing diseases associated with cholesterol dysregulation and may reveal additional therapeutic targets within the mTORC1 signaling pathway.