Alzheimer's disease (AD) is a devastating neurodegenerative condition that ranks as the most prevalent form of dementia among the elderly. With approximately 44 million individuals affected globally, the prevalence is projected to double by 2050, necessitating urgent advancements in diagnostic techniques. A promising avenue of research focuses on utilizing advanced imaging methods, particularly Diffusion Tensor Imaging (DTI), to capture subtle microstructural changes that traditional imaging techniques, like MRI, often overlook.
A recent study conducted by N. Zayed, G. Eldeep, and I.A. Yassine explores the efficacy of DTI in diagnosing Alzheimer's disease. Their work, based on data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), employs various DTI metrics, including Fractional Anisotropy (FA), Mean Diffusivity (MD), and Radial Diffusivity (RD), to delineate the progression of AD and facilitate accurate diagnosis. These measures are designed to reveal the structural integrity of brain tissues, which can be disrupted in AD.
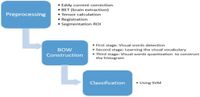
Understanding the significance of this study hinges on its innovative computer-aided diagnosis (CAD) framework, which utilizes image processing techniques to build AD-specific signatures from the hippocampus—an area critical for memory and severely impacted by the disease. By incorporating Scale-Invariant Feature Transform (SIFT) and Speeded Up Robust Features (SURF), the authors aimed to develop a robust system capable of classifying AD, mild cognitive impairment (MCI), and healthy controls effectively.
As Alzheimer’s disease progresses, its symptoms can evolve from mild cognitive impairment to severe mental decline, indicating the pressing need for early diagnosis and intervention. Traditional MRI assessment tends to focus on macro-structural atrophy and often fails to identify critical microstructural changes within the brain's white matter and pathways. The study emphasizes that DTI has the potential to revolutionize this landscape by discerning tissue differences at the micro-level, aiding clinicians significantly in early-stage diagnosis.
The dataset analyzed includes 96 participants: 35 patients with AD, 6 with Early Mild Cognitive Impairment (EMCI), 24 with Late Mild Cognitive Impairment (LMCI), and 31 cognitively healthy elderly individuals serving as controls. Notably, the proposed method achieves an impressive classification accuracy rate of 95.2% when fusing features from MD, FA, and RD maps. Moreover, using FA features for binary discrimination yielded an extraordinary accuracy of 97.5%.
Promising preliminary results demonstrate the potential of the proposed system as a useful tool to capture the AD leanness with achieving accuracies of 87.5%, 87.4%, 89%, and 95.2% for MD, FA, RD, and fusion of features respectively for the multiclass system using SIFT features. The authors state that their approach encapsulates the significant potential of DTI in diagnosing Alzheimer’s disease early, which could allow for timely intervention and improved outcomes for patients.
Furthermore, the innovative use of DTI metrics, particularly RD maps—which have been less utilized in previous research—show a new diagnostic potential, indicating shifts in water diffusion patterns that signify changes in brain microstructure. The significant role of the hippocampus's shape and integrity as a cornerstone in AD progression underscores the relevance of the study's findings, as these changes could serve as critical biomarkers for phase determination of the disease.
In conclusion, the study paves the way for future research utilizing advanced imaging techniques and machine learning approaches. As technologies in brain imaging continue to evolve, the integration of data science with clinical practices holds the promise of not just enhancing diagnostic accuracy, but also tailoring patient treatment strategies to improve quality of life.