Researchers have established a groundbreaking in vitro model to study the respiratory tract of red foxes, a species increasingly recognized for its role in harboring zoonotic pathogens. This model, known as an air-liquid interface (ALI) organoid, allows for a detailed examination of airway epithelial cells and their immune responses, which is crucial given the rising concerns about diseases that can transmit from wildlife to humans.
The research, published on March 22, 2025, in the journal Scientific Reports, focuses on understanding the airway epithelium—an essential barrier against pathogens in the upper respiratory tract. Red foxes (Vulpes vulpes) are of particular interest because they can carry various zoonotic pathogens, including rabies and the fox tapeworm. Given their urban presence and potential health risks, this study aims to advance knowledge on how these animals interact with pathogens.
Conducted in the Swiss canton of Zurich, the study involved isolating respiratory epithelial cells from twelve red foxes hunted during population control efforts. Histological evaluations confirmed that the resultant cell cultures developed a structurally differentiated, pseudostratified epithelium, showing features such as ciliated cells, mucus secretion, and tight junctions that are essential for barrier function.
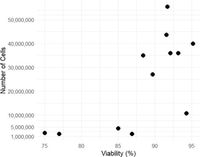
The researchers achieved a high success rate with their culture techniques, yielding between two to 55.5 million cells per fox, with an impressive viability high of approximately 88.3%. This foundational step set the stage for assessing the model's functionality in mimicking in vivo conditions.
One critical assessment performed was transferring epithelial cultures to the ALI system, which produced a significant measure of transepithelial electrical resistance (TEER) at 2,373.9 ± 756.6 Ωcm². TEER is a key indicator of barrier integrity in epithelial layers, and the recorded values suggest that the fox model provides a realistic physiological environment.
To evaluate the immune responsiveness of the fox airway epithelium, the scientists exposed cultures to various stimuli, including lipopolysaccharide (LPS)—a component of bacterial cell walls, which typically triggers an immune response. Remarkably, at four hours after exposure, TNF expression increased dramatically with a fold change of 51.9, indicating an active response mechanism to potentially harmful agents.
However, the models also revealed varied responses to different stimuli. For instance, after 24 hours, TNF expression decreased to a fold change of 3.2 following LPS exposure, while lesser reactions were observed with other stimuli such as phorbol-12-myristate-13-acetate (PMA) and nematode antigens. This provides insight into the innate immune mechanisms active within the red foxes’ airways, highlighting the complexity of their responses to pathogens.
The study underscores the significant advancements that these ALI organoid models represent for the field of respiratory research, particularly regarding less commonly studied species like the red fox. The in vitro model will facilitate better understanding of how wildlife can play a role in disease transmission and the immune responses that underpin these interactions.
Moreover, the research addresses a crucial gap in the existing literature, which often lacks well-differentiated in vitro models for wildlife species. Establishing such models not only aids in the study of host-pathogen interactions but also opens avenues for better public health strategies and veterinary responses to wildlife-transmitted zoonoses.
In conclusion, the newly developed red fox ALI model not only enhances the understanding of respiratory biology in this important species but also serves as a versatile platform for advancing research into zoonotic diseases, thereby highlighting the interconnectedness of animal and human health.