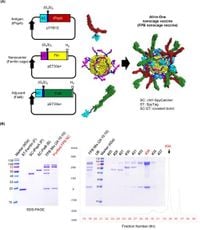
Researchers at Chonnam National University have developed an innovative ferritin-based nanocage vaccine that significantly enhances immune responses against the bacterium Streptococcus pneumoniae, a leading cause of pneumonia worldwide.
The new vaccine leverages a sophisticated delivery system that employs protein nanocages, which can mimic the molecular structure of pathogens. This advancement presents a promising alternative to existing vaccines, potentially addressing the shortcomings of current pneumococcal vaccines.
S. pneumoniae is notorious for its role in severe infections, particularly in children and the elderly, causing approximately 1.2 million deaths globally each year. While traditional pneumococcal vaccines provide some protection, they often fail to induce robust immune memory, particularly in vulnerable populations. Existing subunit vaccines have limited effectiveness as they do not fully utilize the immune system’s capabilities.
The researchers' novel approach involved creating a ferritin nanocage vaccine that displays both the protective antigen tPspA and the mucosal adjuvant FlaB. This combination is designed to activate the immune system more effectively. Using a method known as the SpyTag/SpyCatcher system, the researchers were able to assemble these components efficiently within nanocages.
According to the study, "Our study thus demonstrates the effectiveness of an all-in-one nanocage mucosal vaccine platform, which guarantees enhanced protection with balanced immune responses," wrote the authors of the article. This point emphasizes the nanocage's ability to stimulate a stronger and more comprehensive immunological reaction.
In preclinical trials, BALB/c mice were immunized intranasally with the ferritin nanocage vaccine, and the results were impressive. The vaccine enabled more robust mucosal immune responses than previously developed vaccines, leading to higher levels of secretory IgA—an essential antibody for defending mucosal surfaces.
Subsequent analyses revealed that mice vaccinated with the nanocage exhibited significantly higher production of IgA antibodies, as well as long-lived plasma cells and memory B cells compared to those given the FlaB-tPspA fusion protein.
The effectiveness of the new vaccine was further evaluated through challenge experiments where vaccinated mice were exposed to lethal doses of S. pneumoniae. Remarkably, 100% of mice vaccinated with the ferritin nanocage survived these challenges, compared to only a 55.6% survival rate for those immunized with the traditional FlaB-tPspA vaccine.
A significant aspect of this research is its potential implications—not only for pneumococcal infections but also for developing mucosal vaccines against a variety of pathogens. The advances made in the nanocage vaccine platform could be adapted to target other infectious diseases.
As researchers strive to develop better vaccines that elicit stronger and longer-lasting immune responses, this study underscores the importance of innovative approaches in vaccine design. With the potential to enhance mucosal antibody responses and protection against dangerous pathogens, the ferritin nanocage vaccine represents a significant step forward in the field of immunology.
This breakthrough may propel future research into similar delivery systems, ultimately aiming for more effective vaccines that can safely and effectively protect against a range of infectious diseases. The findings of this study demonstrate that the application of protein nanocages in vaccine development could offer ground-breaking solutions in our ongoing battle against respiratory pathogens like S. pneumoniae.