In a breakthrough that merges nanotechnology with electromagnetic fields (EMWs), researchers have found a promising new strategy to fight antibiotic-resistant infections. The study sheds light on how EMWs below 300 Hz can significantly enhance the antibacterial effectiveness of Graphene Oxide Nanoparticles (GONPs) against the notorious pathogen Pseudomonas aeruginosa, which presents a formidable challenge in the realm of antibiotic resistance.
The prevalence of antibiotic-resistant bacteria continues to rise, necessitating the development of innovative therapeutic solutions. Current treatments are becoming increasingly ineffective against pathogens like P. aeruginosa, which are notorious for their resilience and ability to form biofilms. Biofilms are clusters of microorganisms that adhere to surfaces and are notoriously difficult to eradicate, often leading to persistent infections.
This research, led by a team of scientists, focuses on the properties of GONPs, which are notable for their sharp edges and large surface areas. These nanoparticles, synthesized through glucose pyrolysis, provide a significant advantage due to their ability to disrupt bacterial cell membranes, generate reactive oxygen species (ROS), and interfere with biofilm formation. The method of synthesis involved heating glucose to 250 °C and treating it with a 12.5% ammonia solution, allowing the formation of GONPs that were thoroughly characterized using advanced spectroscopic techniques.
The study delves into how low-frequency EMWs can facilitate the penetration of GONPs into bacterial cells, substantially increasing ROS production and aiding the disruption of biofilms. The researchers meticulously optimized exposure parameters such as frequency, intensity, and duration of EMWs to evaluate their impact on antibacterial activity.
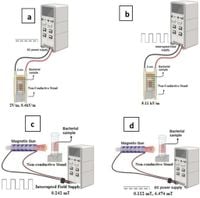
Applying a direct current power supply, the research team established two different exposure protocols. One involved creating electric fields of 2 V/m and 0.4 kV/m, while the other utilized a pulsed magnetic field approach, with a focus on frequencies of 0.7 Hz and 50 Hz. These methods helped establish a controlled environment for testing the combined effects of EMWs and GONPs on bacterial viability.
The findings were significant. When GONPs were exposed to the optimized EMW parameters, the bacteria experienced a reduction in viability exceeding 90%, particularly in the test groups exposed to pulsed magnetic fields. This marked improvement in antibacterial effects indicates that the synergistic combination of GONPs and EMWs creates a powerful new avenue for antimicrobial therapy.
The research demonstrated that the minimum inhibitory concentration (MIC) of GONPs against P. aeruginosa was determined to be greater than 0.039 µg/ml, while the minimum bactericidal concentration (MBC) was noted at more than 0.078 µg/ml. Notably, GONPs leveraged the properties of EMWs to enhance their own absorption into bacterial cells, overcoming conventional cellular defenses.
Further experiments involving growth kinetics revealed that the maximum inhibition rates occurred when the bacteria were subjected to both GONPs and the pulsed magnetic fields, validating the proposed hypothesis that EMWs can amplify the effects of antibacterial agents.
In examining cytotoxic effects, the researchers found that exposure to EMWs led to significant bacterial fragmentation and loss of membrane integrity. These alterations were quantitatively assessed by measuring lactate dehydrogenase (LDH) levels and observing heightened tones of protein and nucleic acid leakage. These findings not only corroborate the enhanced antibacterial potential of GONPs in the presence of EMWs but also point towards the possibility of using this hybrid approach for therapeutic applications.
Despite the promising results, the authors of the article emphasized the need for further exploration into the safety and practical implications of this treatment strategy. Essential considerations include potential stability issues of nanoparticles in clinical settings, the effects of long-term EMW exposure on human health, and the necessity for controlled trials to substantiate these findings in real-world scenarios.
The research represents a significant advancement in the quest for effective treatments against antibiotic resistance and chronic infections. With implications spanning from infection control in medical devices to wound healing, this innovative approach holds the potential to redefine antibacterial therapy. As the battle against superbugs intensifies, methods that incorporate both nanotechnology and EMWs could prove invaluable in developing new and robust medical solutions.