Midlife obesity is becoming increasingly prevalent, and recent research has identified key cellular mechanisms that may explain this growing health crisis. Middle-aged individuals are particularly susceptible to obesity, exhibiting a 22% higher risk of all-cause mortality, but the underlying mechanisms remain poorly understood. New findings highlight the role of adipose progenitor cells (APCs) in the white adipose tissue (WAT) of middle-aged individuals as potential drivers of obesity.
The study published by researchers from Tongji Medical College investigates the extracellular vesicles (EVs) derived from APCs in the context of aging. These EVs are found to have a diminished capacity to counteract the chronic low-grade inflammation, or "inflammaging," that characterizes middle-aged adipose tissue. This dysfunction can lead to enhanced inflammation and insulin resistance, particularly in response to a high-fat diet.
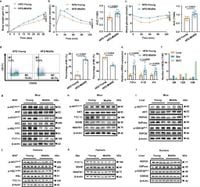
Middle-aged adults aged 30-49 show an alarming 43% obesity rate in the United States, with similar trends worldwide. Despite various lifestyle factors, APCs play a crucial role as they undergo functional decline as individuals age. The study reveals significant reductions in the effectiveness of APC-derived EVs to suppress pro-inflammatory macrophage activity, which is exacerbated by a marked decline in the levels of miR-145, a microRNA crucial for regulating inflammation.
In this research, scientists utilized bulk RNA sequencing and single-nucleus RNA sequencing, revealing that the decline of signaling molecules within the EVs from midlife APCs led to an increase in the polarization of M1 macrophages within WAT. This shift towards a pro-inflammatory state worsens insulin sensitivity. Notably, targeted liposomal delivery of miR-145 mimics effectively mitigated the obesity facilitated by macrophage-driven inflammation in middle-aged mice.
To explore how these APCs alter inflammatory responses, researchers focused on miR-145, which may inhibit macrophage polarization by targeting L-selectin (SELL), a molecule that promotes inflammation. Their findings are significant; as echoed in their findings, "miR-145 in APC-derived extracellular vesicles improves inflammaging and insulin resistance in middle-aged subjects," wrote the authors of the article. This highlights the potential for miR-145 to serve as a therapeutic target for combating obesity in clinical settings.
Methodologically, the study engaged mouse models, employing a high-fat diet to challenge middle-aged mice and assess how EVs from young versus midlife APCs impacted metabolic health. This innovation led to the identification that while EVs from young APCs effectively suppressed inflammation, those from midlife APCs were less capable, thus directly linking age-related changes in APC functionality to midlife obesity.
Researchers identified that upon challenges such as a high-fat diet, middle-aged mice displayed greater weight gain compared to younger counterparts, along with impaired glucose tolerance. This was corroborated by flow cytometry, which evidenced a notable increase in pro-inflammatory cytokines and macrophage populations within adipose tissue, emphasizing the cellular interaction and inflammatory milieu's role in driving obesity.
As the study draws conclusions, it does so by proposing exciting therapeutic avenues. Cationic liposomes were developed for targeted delivery of miR-145 mimics, leading to reduced inflammation and obesity outcomes in midlife mice. Investigators asserted, "APC-derived EVs modulate the risk of midlife obesity, suggesting that miR-145 could be a viable target to combat midlife obesity in clinical settings," highlighting the dual promise of the findings—to understand the mechanisms behind obesity and identify potential interventions.
Looking forward, this research provides important direction for future studies aimed at phosphorus uninstalling aging-related obesity prompts. The integration of cellular biology with therapeutic development could pave the way for innovative approaches to manage obesity and its associated health risks within the rapidly expanding population of middle-aged adults. As obesity rates continue to soar globally, understanding the mechanisms that underlie midlife weight gain is paramount for developing targeted, effective treatments that address not only the symptoms but the root causes of obesity.