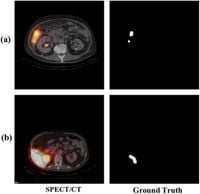
The rapid advancements in medical imaging have heralded a new era in the precision of treatments for liver cancer, one of the most deadly forms of cancer worldwide. Researchers have recently introduced an innovative deep learning model called the Paired Multi-Scale Attention Network (P-MANet) designed to improve the accuracy of liver tumor segmentation through Single-Photon Emission Computed Tomography/Computed Tomography (SPECT/CT) images.
Despite the effectiveness of SPECT/CT in visualizing tumor locations, challenges such as spill-out effects can distort the perception of tumor size, complicating diagnosis and treatment planning. Traditional methods often struggle to accurately delineate tumor boundaries, which are critical for effective treatment strategies such as Selective Internal Radiation Therapy (SIRT). This treatment involves blocking the arterial supply to tumors using radioactive particles, which makes precise tumor mapping essential.
The research, carried out at Chulalongkorn University and King Chulalongkorn Memorial Hospital, trained the P-MANet model on a dataset comprising 43 cases of 99mTc-MAA SPECT/CT scans. The model was specifically designed to address common image quality issues in SPECT/CT, including variations in spectral light that can lead to false positives or negatives during tumor detection.
P-MANet employs a dual-branch architecture: the first branch uses a Multi-Scale Attention Network (MA-Net) to analyze SPECT/CT data, while the second branch applies a White Top-Hat Transform to enhance image features. This innovative technique significantly mitigates effects caused by spectral light variations, which can obscure tumor boundaries, thereby contributing to improved accuracy in tumor segmentation.
According to the study data, P-MANet achieved a Dice Similarity Coefficient (DSC) of 67.93% for normal spectral light distributions and 66.56% for abnormal distributions, yielding an average DSC of 67.00%. The use of advanced deep learning architectures marks a significant improvement in the realm of medical imaging, representing a leap forward from previous methods derived from traditional image processing.
Deep learning, particularly convolutional neural networks (CNNs) like U-Net, has revolutionized medical image segmentation by allowing systems to learn from raw image data. Research highlights that methodologies relying on CNNs exhibit superior performance compared to standard threshold-based techniques, which require manual intervention and can produce inconsistent results. P-MANet builds upon previous innovations like MA-Net and introduces enhancements in feature extraction, allowing for better handling of complex imaging scenarios.
Through rigorous training using advanced augmentation techniques, the P-MANet model was tested against 2,685 images from patients who underwent 99mTc-MAA SPECT/CT scans from 2016 to 2021. The Institutional Review Board approved this study (IRB No. 516/64) to ensure ethical compliance in data collection. The training also involved a significant portion of images, specifically around 33.78%, classified as abnormal due to varied lighting conditions.
One of the pivotal findings of this study centers on how well P-MANet extracts true positives while minimizing false positives. The incorporation of the White Top-Hat Transform in the second branch proved crucial in reducing errors where spectral light was dominant, thereby improving the sensitivity of the model. As noted by the authors of the article, 26ldquo;the cross-features technique for all layers in P-MANet extracts and combines features from multiple scales, significantly contributing to liver tumor segmentation accuracy.26rdquo;
The performance of P-MANet outshone many previous models when evaluated and provided promising implications for SIRT planning, where precise segmentation can lead to improved patient outcomes. The model provides a foundation for further research into improving segmentation accuracy in clinical practice, bridging the gap between imaging technology and effective treatment delivery.
As the researchers concluded, while P-MANet yielded significant results, further optimizations would further enhance its applicability in clinical settings. Strategies such as addressing the model’s focus on reducing false positives and better feature extraction techniques are essential for enhancing segmentation performance. Ultimately, the pursuit of precision in liver cancer treatment hinges on innovative approaches like P-MANet, reinforcing the role of deep learning in revolutionizing medical imaging and therapy.