Researchers in fuel cell technology have unveiled a novel core/shell nanoparticle catalyst that addresses significant efficiency and durability challenges faced by proton exchange membrane fuel cells (PEMFCs). The newly designed catalyst, named PNAC-o-Pt3Fe/C, comprises a Pt3Fe intermetallic core enveloped by an atomically-thin, porous nitrogen-doped carbon layer. This innovative structure is set to enhance performance, especially in reducing the poisoning effects caused by ionomers commonly used in fuel cell applications.
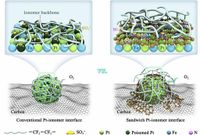
PEMFCs have gained popularity due to their high energy conversion efficiency and low environmental impact. However, researchers have noted that the performance of platinum-based membrane electrode assemblies (MEAs) still falls short of expectations, especially regarding short-term power density and long-term stability. One of the major culprits is the interaction between solid ionomers and the platinum catalyst, which can lead to high resistance and reduced activity of the catalyst sites. To address this issue, the research team implemented a sandwiching strategy involving a core/shell structure that separates the catalyst from detrimental ionomer interactions.
The synthesis of PNAC-o-Pt3Fe/C involved an oleylamine carbonization strategy that utilized platinum acetylacetonate and iron acetylacetonate as metal precursors. The team achieved a uniform dispersion of Pt3Fe nanoparticles, measuring about 4.0 nm in size, supported on the porous carbon. Notably, the added carbon layer not only shields the platinum atoms from ionomer toxicity but also aids in facilitating mass transfer, ensuring better oxygen accessibility at the cathode interface.
Electrochemical testing revealed significant gains in performance metrics. The newly designed catalyst demonstrated a peak power density of 1.0 W cm-2 in H2-air fuel cells. It also achieved a current density of 1.8 A cm-2 at 0.6 V, a remarkable improvement compared to traditional platinum-based catalysts, which often struggle under similar conditions. Importantly, the PNAC-o-Pt3Fe/C not only surpassed its competitors in activity but also proved its durability, exhibiting only a 19.1% activity degradation during a 100-hour constant potential test.
Through tests under intensive operational conditions, the combination of the atomically-thin carbon layer and the embedded Pt3Fe core was shown to restrict the negative impact of ionomers, minimizing the coverage of sulfonate groups on the active platinum sites to just 10.1%. In comparison, the coverage was noted to be 21.2% and 23.3% for other catalysts without this protective layer. The electrochemical active surface area (ECSA) of PNAC-o-Pt3Fe/C recorded an impressive 60.3 m2 gPt-1, which closely approaches its theoretical maximum.
Significantly, the team noted that this unique catalyst retains its intermetallic structure and stability under electrochemical cycling, with only a minor voltage shift of 2 mV after 60,000 accelerated aging cycles. Such robustness illustrates the capability of the carbon layer to safeguard Pt3Fe nanoparticles from agglomeration and preserves their catalytic efficiency even under severe operation conditions.
By integrating a porous N-doped carbon layer with the Pt3Fe core, the researchers have set a new benchmark for enhancing catalyst designs in PEMFCs, potentially leading to advancements in fuel cell technology for sustainable energy applications. Future research will focus on scaling this synthesis process and further improving the catalytic properties as PEMFCs continue to evolve toward energy efficiency and environmental sustainability.