Recent advancements in electrochemical processes have led to the development of a groundbreaking method for producing acetate from carbon monoxide (CO) using a modified copper catalyst. Researchers at the University of Science and Technology of China have harnessed the capabilities of cerium single atoms (Ce-SAs) to enhance the efficiency of copper-based catalysts, making it a significant step forward in sustainable chemistry.
Traditional methods of acetate production are often energy-intensive and accompanied by substantial carbon emissions, largely due to the prevalent thermal carbonylation processes. However, this new approach utilizes renewable electricity to drive the electrochemical conversion of CO into valuable chemicals, positioning itself as an environmentally friendly alternative.
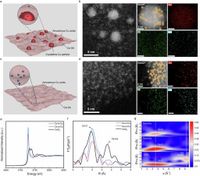
The novel catalyst designed in this study features a crystalline-amorphous dual-phase copper matrix modified with Ce-SAs. This configuration plays a crucial role in regulating the electron density on the catalyst surface, which is pivotal in controlling the reaction pathways. Specifically, the modified catalyst promotes the formation of a ketene intermediate, which subsequently reacts with hydroxide ions generated through pulsed electrolysis to yield acetate.
The team reported impressive results: achieving a Faradaic efficiency (FE) of 71.3 ± 2.1% for acetate production, along with a time-averaged acetate current density of 110.6 ± 2.0 mA cm−2. This level of efficiency in converting CO to acetate marks a significant improvement over existing methods.
“By utilizing Ce-SAs, we have unlocked new pathways for improving the selectivity of CO electrolysis towards acetate production,” said M.-R. Guo, the leading researcher of the study. The findings illustrate that through careful modification of catalyst design, it is possible to reduce the competition from undesired hydrogen evolution reactions that typically hinder acetate formation.
The dual-phase copper catalyst operates effectively in a pulsed electrolysis mode, demonstrating not only high selectivity but also remarkable stability. The catalyst maintained its performance for over 138 hours with a selectivity greater than 60%, showcasing its potential for long-term applications in chemical manufacturing.
The incorporation of Ce-SAs was found to significantly lower the coverage of hydrogen intermediates on the catalyst surface, consequently favoring the selective formation of acetate. This innovative strategy minimizes the formation of competing products like ethylene, which has been a persistent challenge in CO-based catalytic processes.
In operational stability tests, the Ce-SAs modified dual-phase copper catalyst showed no evidence of performance degradation, further solidifying its viability for industrial applications. Such attributes are essential for the commercial scaling of electrochemical CO-to-acetate technology, which could play a pivotal role in carbon capture and utilization frameworks.
The research illustrates that not only does the choice of catalyst significantly affect the electrochemical conversion process, but so does the environmental condition under which the reactions are conducted. By transitioning from traditional alkaline environments to pulsed electrolysis conditions, the team effectively improved acetate production rates. Additionally, operating under a pulsed regime has been shown to enrich hydroxide concentrations, further propelling reaction efficiencies.
Moreover, the study highlights the potential applications in the production of acetic acid, a critical chemical used in various industries, including textiles, pharmaceuticals, and food packaging. Current industrial sources of acetic acid are predominantly linked with high carbon emissions, thus emphasizing the importance of adopting greener production methods as exemplified in this research.
Looking forward, the authors believe that scaling up this technology could substantially decrease reliance on fossil fuels while contributing to greenhouse gas mitigation strategies. With the ability to operate efficiently using renewable sources of electricity, this electrochemical approach offers a promising avenue for sustainable chemical manufacturing.
While the results are encouraging, the researchers acknowledge that further studies will be necessary to completely understand the long-term durability and efficacy of the Ce-SAs modified copper catalyst under varied reaction conditions. The ongoing research aims to explore the full capacity of this innovative catalyst design, with the hope of advancing it to commercial viability.
In conclusion, the Ce-SAs modified dual-phase copper catalyst represents a significant scientific advancement in the electrochemical reduction of CO to acetate, showing promise not only for acetate production but also as a model for future research into catalyst design and functionalization in the quest for sustainable chemical processes.