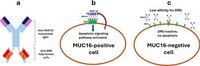
The development of a novel bispecific antibody, IMV-M, represents a significant breakthrough in cancer therapy. This innovative treatment is designed to selectively bind and cluster death receptor 5 (DR5) on cancer cells, specifically targeting tumors that express the antigen MUC16, which is commonly overexpressed in numerous aggressive cancers including ovarian, pancreatic, and lung cancers.
Researchers have found that IMV-M effectively induces anti-tumor activity without the need for secondary crosslinking, meaning it can function independently and achieve tumor cell death more efficiently. Laboratory testing has demonstrated that IMV-M can kill MUC16-positive cancer cells at remarkably low concentrations, showcasing its potential for broad therapeutic application with a more favorable safety profile compared to conventional antibody-drug conjugates (ADCs).
The cornerstone of IMV-M's innovative approach lies in its unique design, which features a high-affinity anti-MUC16 antibody fused with a low-affinity DR5 antibody. This structure allows multiple IMV-M molecules to cluster around a single MUC16 molecule, significantly increasing the clustering of DR5 receptors on tumor cells, which is pivotal for activating the apoptosis (programmed cell death) pathways.
In vitro experiments involving a range of human MUC16-positive cancer cell lines showed that even the lowest tested doses of IMV-M could effectively inhibit tumor cell proliferation and induce apoptosis within hours of exposure. For instance, in a pancreatic cancer cell line, treatment with only 0.16 nM of IMV-M was sufficient to eradicate cell populations. This level of potency is noteworthy, offering new hope for patients who may not respond to existing treatments.
The efficacy of IMV-M extends beyond laboratory settings; in animal models, particularly xenografts implanted with MUC16-positive pancreatic adenocarcinoma cells, a single infusion of 5 mg/kg resulted in significant tumor regression. In fact, researchers observed that IMV-M displayed robust anti-tumor activity across multiple xenograft models, demonstrating effectiveness even at doses as low as 1 mg/kg.
Importantly, the safety profile of IMV-M has been promising. In studies conducted with cynomolgus monkeys, administration of the antibody at varying doses revealed no signs of toxicity, suggesting that IMV-M is well-tolerated and safe for further clinical evaluation. This aspect is crucial as existing therapies often come with severe side effects, limiting their use in clinical settings.
IMV-M's mechanism of action distinguishes it from traditional ADC therapies that rely on cytotoxic compounds. Instead of utilizing a toxic payload that can harm healthy tissues, IMV-M engages directly with DR5 and MUC16 to trigger cell death, significantly reducing the risk of collateral damage to normal cells. This targeted approach allows IMV-M to act selectively on tumors while sparing healthy tissue, a quality that is increasingly sought after in oncology therapeutics.
With the prevalence of MUC16 expression in many types of cancer, from pancreatic to ovarian tumors, IMV-M provides a promising avenue for tackling malignancies that currently have limited treatment options. The research results underscore the potential of IMV-M as an innovative and safe therapeutic candidate, paving the way for future clinical trials aimed at evaluating its efficacy and safety in human patients.
“Our findings suggest that antibody clustering effectively induces DR5 clustering, resulting in anti-tumor activity,” wrote the authors of the article, emphasizing the novel mechanism through which IMV-M operates.
In summary, IMV-M represents an exciting advancement in cancer treatment, particularly for patients suffering from tumors that overexpress MUC16. As research continues to unfold, IMV-M could very well become a cornerstone in the future of targeted cancer therapies.