A novel artificial enzyme, dubbed Zn(II)-SMM, has been developed for the hydrolysis of p-nitrophenyl acetate (PNPA) with remarkable efficiency, showcasing catalytic rates exceeding 5239 times those of traditional non-catalytic systems. This new enzyme presents significant promise for mimicking the catalytic capabilities of natural enzymes, thereby opening doors to efficient industrial applications.
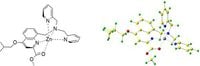
The study, published on March 18, 2025, details the innovative design of this small molecular enzyme complex. By integrating hydrophobic quinoline and dipyridinium within the Zn(II)-SMM framework, researchers enhanced the binding affinity between the enzyme and its substrate, enhancing overall efficacy. With the use of the Zn2+ ion acting as the catalytic metal center, Zn(II)-SMM efficiently catalyzes the hydrolysis of PNPA, conforming to established Michaelis-Menten kinetics.
Historically, the pursuit of synthetic enzymes has yielded varying degrees of success. Traditional hydrolysis reactions often relied on complex structures characterized by rigidity and limited application scope. The Zn(II)-SMM system sets itself apart through its flexibility, enabling the optimization of both catalytic and substrate binding sites. This advancement allows for broader application in various chemical reactions and biological processes.
To assess its catalytic efficacy, the research team experimented with different concentrations of PNPA, observing significantly increased reaction rates when compared to spontaneous reactions. This confirms the Zn(II)-SMM’s surprising efficiency, which consistently completes hydrolysis reactions within approximately sixty seconds.
The phenomenon of reaction rates increasing upon elevational concentration of the substrate highlights the enzyme’s exceptional performance. This activity was quantified using both experimental and computational methods, and the resulting kinetic parameter value was determined to be 0.8287 min-1 (Kcat).
Zn2+ is observed to adopt a planar structure involving acyl oxygen and nitrogen atoms, achieving maximum stability within a tetrahedral configuration, which is energetically favored. This coordination inspires confidence within the research community about the enzyme's reliability and potential for real-world applications.
Throughout the study, UV-vis spectroscopy served as another integral tool, allowing researchers to monitor phenol production during the hydrolysis of PNPA, which displayed notable UV absorption at 400 nm. Such insights reveal not only the immediate catalytic effects but also validate the enzymatic attribution to Zn(II)-SMM.
The kinetics of the catalytic activity and the underlying mechanisms are sufficiently elaborated upon. A characteristic double reciprocal plot confirmed adherence to Michaelis-Menten dynamics. This suggests enzyme-like characteristics of the Zn(II)-SMM complex, providing insights necessary for future advancements.
The authors of the article assert, "The newly constructed small molecular enzyme complex Zn(II)-SMM exhibits surprisingly high activity for the hydrolysis of PNPA, consistently concluding reactions within approximately sixty seconds." This statement encapsulates the essence of their findings, reflecting the system’s efficiency.
Research findings aid the quest to develop small molecular hydrolases which possess significant catalytic activity akin to natural enzymes. The exploration of the hydrolysis mechanism reveals how the activation of Lewis acid forms within the Zn(II)-SMM complex facilitates optimal catalytic interactions.
Notably, the hydrolysis occurs more efficiently under neutral conditions, and the mechanism aligns with single Lewis acid activation hypotheses. Therefore, future investigations may build upon this study by exploring the design of chiral metallohydrolases, potentially revolutionizing synthetic enzymatic applications.
Conclusively, the novel Zn(II)-SMM enzyme symbolizes a significant advancement within synthetic enzymology. The successful development of this complex not only enhances our comprehension of catalytic systems but also paves the path for future innovations within the field of metal hydrolases.