Fibrosis is caused by pathological activation of resident fibroblasts to myofibroblasts, leading to tissue stiffening and organ dysfunction. Despite its prevalence, methods for grading fibroblast phenotypes are often subjective. A new study introduces a quantitative approach using mathematical descriptors of cell morphology and intracellular structures to characterize these transitions more effectively.
This revolutionary analytical framework employs advanced statistical modeling to reveal key features associated with fibroblast phenotypes. The researchers trained and validated their models using data from over 3,000 primary heart valve interstitial cells (VICs). Importantly, this approach proves invaluable for screening potential therapeutics aimed at treating fibrotic diseases.
Fibrosis, which occurs in various organs including the heart, kidneys, and lungs, leads to approximately 45% of deaths globally. Current treatment options remain limited. The study notes that while two drugs are approved for idiopathic pulmonary fibrosis, the complexities surrounding the initiation and progression of such diseases hinder effective therapy development.
Researchers focused on the fibroblast-myofibroblast transition, a key event in fibrosis. As these cells undergo pathological changes, they produce excess extracellular matrix components, leading to tissue rigidity and impaired function. Traditionally, visual assessments and subjective grading methods relying on immunostaining with alpha-smooth muscle actin (alpha-SMA) have been utilized, but these methods often lack precision.
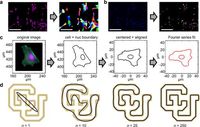
In their innovative study, the researchers utilized Fourier series analysis to describe cellular and nuclear shapes mathematically, allowing for a more objective assessment of fibroblast activation. In addition, they focused on coherence measurements of alpha-SMA stress fibers to quantify the organization of these proteins, offering a continuous spectrum of quiescent to activated states.
One key innovation of this research is a new descriptor known as the degree of anisotropy (DOA), which quantifies the organization of alpha-SMA stress fibers and aligns with traditional grading practices. This allows for a more refined categorization of fibroblast phenotyping based on their structural characteristics.
The researchers mixed two methods - a multivariable logistic regression model and the mathematical descriptors they developed - to achieve a better predictive capability for identifying reactive fibroblasts. Their findings suggest strong accuracy with the DOA feature reaching up to 83% in predictive tasks, outperforming traditional methods significantly.
By comparing their methods with manual grading, logistic regression models were trained that achieved impressive predictive performance as well. Their validation process called k-fold cross-validation confirmed that cell and nuclear areas combined with DOA could accurately grade VICs.
Moreover, upon testing their models with the small molecules 5-azacytidine (AZA) and bisperoxovanadium (bpV(HOpic)), their approach demonstrated a promising ability to predict drug responses. The results indicated that AZA treatment, which promotes healthy fibroblast states, significantly influenced the activation status of VICs and suggest that BPV(HOpic) induces myofibroblast activation shown through a substantial increase in activated VICs.
Overall, this study presents a notable leap in fibrotic disease research, providing a robust framework for more reliable categorization of fibroblast phenotypes. This shift from traditional subjective methods to data-driven approaches is expected to enhance drug development and could lead to groundbreaking advancements in understanding and treating fibrosis.
The authors of the article emphasize, "This work introduces an analytical framework that unveils key features associated with distinct fibroblast phenotypes via quantitative image analysis and is broadly applicable for high-throughput screening assays of candidate treatments for fibrotic diseases." The authors foresee their model being used as a standard for grading fibroblasts across various biomedical research studies.
By laying the groundwork for further work in the field of fibrosis, future studies can confidently build upon these results, refining techniques to combat this pervasive health issue more effectively.