Early research into triple-negative breast cancer (eTNBC) has unveiled promising approaches in predicting patient outcomes through the analysis of circulating tumor DNA (ctDNA). In a recent study involving 130 female patients with stage II-III eTNBC receiving neoadjuvant chemotherapy (NAC), researchers aimed to determine the prognostic value of ctDNA in risk assessment ahead of surgical intervention.
Triple-negative breast cancer, known for its aggressive nature and challenging treatment outcomes, comprises patients with a dismal prognosis by standard metrics. The five-year overall survival rate is alarmingly low, and the rapidity of metastasis renders the identification of robust biomarkers essential in managing the disease effectively. This study, published on March 21, 2025, highlights that ctDNA levels, when monitored dynamically throughout treatment, provide critical insights into relapse risks and patient management strategies.
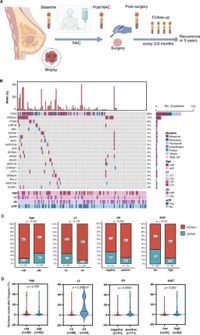
Researchers discovered that ctDNA analysis performed after NAC but prior to surgery, as well as following surgery, correlates strongly with a worse prognosis. Specifically, a threshold of 1.1% maximum variant allele frequency (MVAF) at baseline emerged as a key metric for stratifying patients into differing relapse risk categories. The model integrating both baseline ctDNA levels and post-surgery results proved independently prognostic, with a significance level of p = 0.022, thus indicating its potential utility in tailoring future treatment protocols.
Among the cohort, the median age of participants was 44 years, with a follow-up median of 24.2 months. Notably, of the 122 patients with available baseline blood samples, a high 78.7% exhibited detectable ctDNA levels. After administration of NAC, significantly, the positive ctDNA rate diminished from 79% to 23% prior to surgery, underlining the treatment's efficacy in many patients.
The integration of ctDNA into clinical practice offers a dual advantage: first, it can help highlight a subgroup of patients who have a very favorable response to standard treatments, and second, it delineates a high-risk cohort that may benefit from escalated treatment interventions to avert recurrence. For instance, patients showing ctDNA positivity after surgery exhibited far worse event-free survival (EFS) and distant recurrence-free survival (DRFS) rates, strongly linking ctDNA levels to future cancer outcomes.
More startling are findings associated with longitudinal ctDNA surveillance, revealing that the recurrence was often detectable earlier than conventional imaging techniques would suggest. The study found that upon detection of minimal residual disease (MRD), on average, the mean lead time to clinical relapse was approximately 3.4 months, a significant window for implementing potential therapeutic strategies.
Combining systematic measures of tumor burden with histopathological responses offers a groundbreaking approach to risk stratification in patients with eTNBC. This is especially evident when considering those who achieve pathological complete response (pCR). The study reported that higher systemic tumor burden correlates directly with worse survival rates, ultimately aiding clinicians in deciding between escalation or de-escalation of treatments based on the comprehensive ctDNA analysis.
“Circulating tumor DNA surveillance during follow-up identifies patients with high relapse risk,” noted the authors of the article. This highlights the promising potential of ctDNA not just as a tool for stratification but as a regular part of the eTNBC treatment diagnostic and monitoring strategy going forward.
This research underscores the necessity for ongoing exploration into ctDNA’s role in breast cancer management, particularly for eTNBC, where conventional treatment paradigms often fail to provide satisfactory outcomes. As the results suggest, the elevated threshold of baseline ctDNA provides critical stratification of relapse risk, making it clear that targeted clinical decisions hinge on these biomarkers could lead to individualized and more effective treatment protocols in the future.