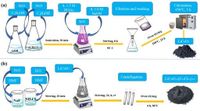
The continuous pursuit for efficient energy storage solutions has led researchers to explore innovative materials for supercapacitors. A recent study from the University of Guilan reveals significant advancements in the performance of supercapacitors by modifying lithium cobalt oxide (LiCoO2) through anionic engineering, showcasing its enhanced capabilities for energy storage applications.
Supercapacitors are increasingly recognized for their high energy density, rapid charging abilities, and long cycle life, enabling them to outperform traditional battery technologies in specific applications. However, achieving a balance between high energy density and stability has remained a challenge. This study hypothesizes that altering the anionic structure within lithium cobalt oxide can unlock substantial improvements in energy density and charge storage capability.
Using a sol-gel synthesis method, the research team created several variants of lithium cobalt oxide modified through anion exchange techniques. Specifically, they synthesized LiCoO2−x(F0.8Cl0.2)x and investigated its electrochemical performance. The results were nothing short of impressive. The anion-exchanged variant, particularly LiCoO1.6(F0.8Cl0.2)0.4, achieved a peak specific capacitance of 522.16 F g−1 at a current density of 1 A g−1, significantly outpacing traditional lithium cobalt oxide electrodes and characterized by superior electrochemical performance.
In their tests, the modified supercapacitor demonstrated a combination of remarkable energy density—93.98 Wh kg−1—and power density of 0.3 kW kg−1 under similar conditions. In addition, it exhibited excellent cycling stability, retaining 92.04% coulombic efficiency after 4000 charge-discharge cycles, a crucial factor in practical energy storage devices.
These findings are rooted in the fundamental properties of lithium cobalt oxide, known for its unique atomic arrangement, which can be tailored at the nanoscale to maximize electrochemical interactions with electrolyte ions. By employing anion exchange through the incorporation of fluoride and chloride ions, the researchers have effectively modified the electronic structure of the material, significantly enhancing electrochemical properties. As explained by the authors of the article, "Anion exchange allows for the partial substitution of anions in mixed metal oxides, thereby enhancing their electrochemical properties." This underscores the potential of anionic engineering as a vital technique in the design of high-performance supercapacitors.
The enhanced capacitive performance of the LiCoO1.6(F0.8Cl0.2)0.4 electrode not only highlights its potential for applications in electric vehicles and portable electronics but also demonstrates the material's versatility in the broader energy storage landscape. The observed maximum specific capacitance of 512 F g−1 places it well above many conventional supercapacitor materials, such as activated carbon and early metal oxides.
Electrode fabrication involved creating working electrodes by blending the synthesized lithium cobalt oxide with conductive materials and applying them onto a nickel foam substrate, ensuring effective electron transport during electrochemical processes. To further evaluate the electrochemical performance of their synthesized materials, the researchers conducted cyclic voltammetry (CV), galvanostatic charge-discharge tests, and electrochemical impedance spectroscopy (EIS). These techniques collectively indicated how well the material performs under operational conditions, providing critical insights into charge storage mechanisms.
According to the study, while the LiCoO1.6(F0.8Cl0.2)0.4 electrode demonstrated superior performance characteristics, the fundamental science behind its performance revealed a need for balance in material properties during modification. The researchers noted that higher concentrations of substituted ions could disrupt the crystal lattice, hampering performance, stressing the importance of optimal compositions in producing effective supercapacitor materials.
In conclusion, the advancements achieved in this study position modified lithium cobalt oxide as a highly efficient candidate for supercapacitor applications, with the authors noting that, "The LiCoO1.6(F0.8Cl0.2)0.4 sample showed a prominent specific capacitance of 512 F g−1 and a coulombic efficiency of 92.04% after 4000 cycles at a current density of 2 A g−1." These developments signal exciting possibilities for future research aimed at creating even more powerful energy storage solutions, which are desperately needed in the global shift toward carbon-neutral technologies.