Atrial fibrillation (AF), the most common cardiac arrhythmia, impacts roughly 33 million people globally and presents significant health risks, including an increased likelihood of stroke. Conventional treatments often involve catheter ablation, a procedure that can lead to substantial side effects. However, a team of researchers at Zhejiang Chinese Medical University has been exploring a novel approach using microsecond pulsed electric fields (µsPEFs) as an alternative treatment modality that promises greater efficacy with fewer adverse effects.
This innovative study specifically examines how µsPEFs can effectively induce cardiomyocyte apoptosis and delves into the mechanisms behind this destruction. The research findings suggest that µsPEFs could revolutionize AF management by providing a safer, non-thermal option for patients. Overall, the study underscores the importance of refining electrical parameters for the most effective cardiomyocyte ablation.
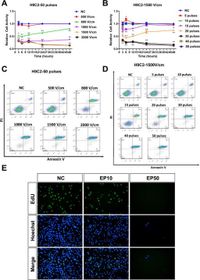
The researchers began by investigating the influence of varying voltage and pulse duration on live H9C2 and HL-1 cardiomyocyte cells in vitro, utilizing assays such as CCK8 and flow cytometry to determine cell activity and death rates. Results indicated a significant decline in cell viability beyond specific electric field thresholds: a voltage of 1500 V/cm paired with a pulse count of 50 resulted in a staggering 95% apoptosis rate.
"Notably, at a voltage of 1500 V/cm and a pulse count of 50, the apoptosis rate exceeded 95%, coupled with a more stable and consistent cell ablation," wrote the authors of the article. This high rate of cell death, coupled with a continuous decline in cell activity over time, confirms the efficacy of µsPEFs as a viable treatment against AF.
The study employed a combination of molecular techniques, including transcriptome sequencing and transmission electron microscopy (TEM) to scrutinize the changes occurring at a cellular level. Significant alterations in gene expression patterns were observed, especially related to mitochondrial function. The analysis revealed upregulation of several genes associated with mitochondrial activity, confirming the method's focus on targeting cardiomyocytes without causing excessive damage to surrounding healthy tissues.
The research also extended to in vivo experiments conducted on mice to reinforce the in vitro findings. Results indicated that µsPEFs contributed to dynamic changes in myocardial cell structure and function. The mice displayed symptoms attributed to cell fragmentation and inflammatory responses immediately following ablation, observable through hematoxylin and eosin (H&E) and Masson staining.
TUNEL assays confirmed the activation of apoptosis pathways, indicating that cardiomyocytes in the ablation zone demonstrated significant susceptibility to the electric pulses. "µsPEFs induce cell injury by impairing mitochondrial function and potentially triggering the mitochondrial apoptosis pathway," noted the authors, highlighting the crucial role of mitochondrial disruption in mediating cell death.
Furthermore, increased levels of Cytochrome C from mitochondria during the apoptotic process were recorded, signaling critical pathways that lead to cell death—reinforcing previous findings surrounding the relationship between electric fields and mitochondrial integrity. This mechanism of action presents an exciting opportunity for the development of new therapeutic strategies targeting AF, which would vastly improve patient safety.
The implications of this research are profound: as AF incidence continues to rise, the ability to employ µsPEFs as an alternative to traditional treatments could alleviate both the health risks associated with the condition and reduce the long-term healthcare costs tied to AF management. Moreover, the findings provide a fresh foundation for further investigations into cardiac ablation techniques.
In summary, the research conducted displays the impressive capabilities of µsPEFs in inducing targeted cardiomyocyte apoptosis, thereby paving the way for new treatment paradigms for atrial fibrillation. Through understanding and refining the parameters that govern this process, healthcare professionals may soon have a powerful new tool in their arsenal against this prevalent arrhythmia.