The use of machine learning to quantify brain imaging signatures related to cardiovascular and metabolic risk factors offers a promising advancement in personalized medicine for assessing dementia risk.
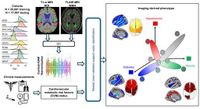
Recent research has unveiled machine learning models capable of detecting refined brain signatures associated with cardiovascular and metabolic risk factors (CVMs), such as hypertension, hyperlipidemia, smoking, obesity, and type 2 diabetes. This study, part of the expansive iSTAGING initiative, harmonized MRI data from over 37,000 individuals drawn from ten international cohort studies to improve early detection of dementia.
Researchers involved in this study aimed to address the gaps in understanding how these CVMs differentially impact brain structure and ultimately cognitive health. The findings are paramount, particularly as age-related risks like these contribute significantly to the global dementia burden.
Through their sophisticated machine learning approach, the team developed five in silico severity markers that outperformed conventional MRI findings. Notably, these markers were not only more sensitive at identifying subtle structural changes in individuals as young as 45 years, but also associated strongly with cognitive function and beta-amyloid deposition, a key indicator of Alzheimer’s disease.
“This study presents a novel approach to quantify CVM-related brain changes using machine-learning-derived MRI markers,” noted the authors of the article. By integrating individualized measurements of CVM impacts, the research can potentially revolutionize how clinicians approach dementia risk and patient management.
For many years, the connection between CVMs and cognitive decline has been noted, yet understanding the precise associations has proven challenging. Traditional diagnostic classifications do not account for the nuanced individual variations present in patients, resulting in a one-size-fits-all approach that may overlook critical variations in risk profiles.
The study leveraged data from diverse geographic and demographic backgrounds to address these challenges. Researchers utilized a mixture of T1-weighted and T2-weighted FLAIR MRI images, employing machine learning algorithms to derive distinct spatial patterns of abnormalities related to each cardiovascular and metabolic risk factor.
From their analyses, it became clear that the markers developed, termed SPARE-CVMs, not only detected changes more effectively than past methods but also effectively captured the broader implications of comorbid CVMs that many patients experience simultaneously.
The SPARE-CVM indices showcased a stronger correlation with cognitive performance than traditional diagnostic methods have shown, underlining a compelling narrative for earlier intervention strategies based on these individualized assessments. Higher SPARE-CVM values coincide with reduced gray and white matter volumes, elucidating the brain's structural response to these risk factors.
“Integrating personalized measurements of CVM-specific brain signatures into phenotypic frameworks could guide early risk detection,” the authors emphasized, advocating for this personalized approach in clinical settings.
As the study highlighted, the most pronounced effects in SPARE-CVM scores were observed in mid-life participants, suggesting that targeting these risk factors in earlier life stages could mitigate future dementia risk. This insight not only enhances our understanding of how age interacts with cvm impacts but suggests a pathway for developing more focused interventions.
The implications of such a study extend beyond individual patient assessments; they could influence the development of next-generation clinical trials targeting these specific risk factors. With the robust findings from this extensive multinational dataset, the researchers call for the incorporation of SPARE-CVMs in future studies.
The ability to associate SPARE-CVMs with cognitive performance also raises critical questions about the broader healthcare implications for populations most affected by these comorbid conditions. Addressing health inequalities shaped by race, ethnicity, and socioeconomic status could ultimately lead to more effective public health strategies as these insights are translated into clinical practice and health policy.
In conclusion, this groundbreaking research establishes machine learning as a vital tool in understanding the complex relationships between cardiovascular and metabolic health and cognitive decline. The nuanced detection of subtle brain changes through SPARE-CVM indices represents a significant leap forward in tackling the rising dementia epidemic. The community awaits further studies to validate these findings, paving the way toward proactive approaches in patient care that account for individual risk profiles, ultimately aiming to reduce the future burden of dementia worldwide.