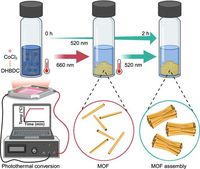
In a groundbreaking study, researchers have harnessed the power of light to assist in the synthesis of metal-organic frameworks (MOFs), leveraging the unique heating properties of photothermal materials. This innovative approach, developed by a team led by Yufeng Wang, is set to revolutionize material synthesis techniques by enabling the remote control of chemical reactions using visible light.
Traditionally, temperature control in chemical reactions has been critical for determining reaction rates and outcomes. The new method utilizes photothermal materials like a cobalt chloride molecular complex to convert light into heat, facilitating the formation and assembly of complex MOF structures. By employing five different converters, including palladium nanoparticles (PdNPs) as competing agents, the researchers were able to explore a wealth of dynamic interactions within the synthesis process.
Published in Nature Communications, the study highlights the versatility of this state-of-the-art approach, using cobalt chloride to achieve a maximum temperature of 140 °C under green light, while a rapid heating to reflux temperature occurred under red light conditions. Such temperature control is essential for mastering the assembly of nanorod structures into superstructures, a capability previously limited by conventional heating methods.
During the experiments, the researchers found that under a green LED (520 nm), rapid nucleation occurred within the first 25–30 minutes. In contrast, applying a 660 nm LED led to the formation of large hexagonal structures after approximately 30-40 minutes. This ability to spur different growth patterns simply by alternating the light source exemplifies the method's remarkable flexibility in guiding material formation.
In total, the project unveiled that using cobalt chloride, the assemblies could grow to lengths of approximately 5000 nm and widths of about 3800 nm, far surpassing the 1200 nm lengths typical of traditional heating methods. This significant enhancement showcases the potential for creating more complex materials with improved properties.
The addition of platinum nanocrystals introduced an intriguing aspect to the study. Their presence influenced the assembly dynamics, with experiments showing that at certain concentrations, palladium can hinder the growth of the MOF structures. Investigating these phenomena may be pivotal for future applications in photothermal materials, especially where precise control over molecular interactions is desired.
This comprehensive study opens a new frontier in material chemistry, illustrating the feasible integration of photothermal techniques. The insights gained into molecular interactions and heating effects can pave the way for advanced synthetic routes, which are crucial for applications across various disciplines such as catalysis, electronics, and drug delivery.
As light-assisted heating continues to gain traction, the teams’ findings potentially herald a paradigm shift in how materials are synthesized and assembled. By capitalizing on the unique characteristics of photothermal materials, scientists can attain levels of control previously unattainable, leading to unprecedented advancements in material science.