Recent research has illuminated the potential of lanreotide, a somatostatin analogue, as an effective treatment for persistent diabetic macular edema (DME) — particularly among patients who have not responded to conventional therapies.
Diabetic retinopathy (DR), primarily caused by diabetes mellitus, is one of the leading causes of blindness globally, responsible for approximately 12% of new blindness cases each year. Among its more severe forms, diabetic macular edema can lead to significant vision loss. Standard treatments typically include laser therapy and anti-vascular endothelial growth factor (anti-VEGF) injections, but challenges remain for patients whose conditions are resistant to these interventions.
A study conducted by Ester Fernandez-Lopez and her colleagues at FISABIO Eye Clinic, Valencia, Spain, focused on investigating the effects of lanreotide on patients presenting with persistent DME. Published on March 17, 2025, this study aims to provide new insights and potential solutions for those suffering from this debilitating condition.
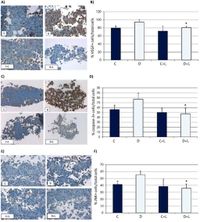
Previously reported treatments for DME have proved insufficient for about 30% of patients, leading to severe and persistent cases of edema. Dr. Fernandez-Lopez’s team explored alternative therapies, proposing somatostatin analogues like lanreotide as promising candidates. Thanks to its properties, lanreotide may inhibit growth hormones and exert protective effects against cell apoptosis (programmed cell death), which contribute to the worsening of DR.
To evaluate lanreotide's efficacy, two distinct studies were performed. The first involved using human retinal pigment epithelial (RPE) cells cultured under controlled conditions, exposing them to varying glucose levels to simulate diabetic conditions. The second study followed 18 patients experiencing persistent DME who had undergone standard treatment methods without success. Participants received subcutaneous injections of lanreotide every four weeks over the course of one year.
The results reflected substantial improvements: patients experienced visual acuity increases of 8.62 letters on average, with 50% showing gains of over 10 letters and nearly 27% improving by more than 15 letters on the ETDRS scale — a standard method for measuring visual acuity.
Central macular thickness, measured throughout the year, decreased significantly by 106 micrometers, with macular volume also showing notable reductions. This improvement suggests effective anatomical changes aligned with the observed visual enhancements. Notably, prior to receiving lanreotide, all study participants had already pursued treatment options involving laser therapy or anti-VEGF injections.
Statistical analyses proved the improvements to be significant, instilling hope for patients lacking responsive options. Past research has highlighted the role of growth hormone and related pathways contributing to the persistence of DME, marking lanreotide as potentially impactful due to its dual anti-inflammatory and antiapoptotic properties. This aligns with previous findings leveraging somatostatin analogues aimed at managing persistent conditions.
Across the study cohort, 85% were reported to have non-proliferative diabetic retinopathy (NPDR), contributing to the complexity of managing their conditions. Most would have traditionally faced limited treatment options but benefited from this clinical trial, showing improvements not just qualitatively but quantitatively, measured both through visual tests and anatomical assessments.
Throughout the year of the clinical trial, patients also experienced significant decreases in total cholesterol, triglycerides, and albuminuria — additional factors known to affect diabetic complications adversely. Such results highlight the multifaceted potential of lanreotide beyond DME treatment alone, promoting overall metabolic health improvements.
Despite several patients dropping out due to adverse effects, the overall tolerance toward lanreotide improved markedly over time, with 77% of patients reporting no systemic adverse effects post-treatment at the end of the year. Previous treatment protocols often yielded unmanageable side effects, rendering patients less enthusiastic toward intervention. Therefore, achieving both therapeutic efficacy and patient comfort with lanreotide could set it apart as a viable option for those needing intervention.
Dr. Fernandez-Lopez stated, "Lanreotide showed antiapoptotic effects and significantly improved visual acuity. It could be applied in non-respondent patients to other treatments as an alternative for refractory DME patients." This encapsulates the essence of the findings and elucidates the necessity for continued exploration and adoption of lanreotide.
Looking forward, the study authors are optimistic about conducting larger trials to examine long-term effectiveness and to establish controlled trial comparisons, aiming for solid scientific ground. Improvement of patient outcomes and quality of life remains the ultimate goal, especially considering the chronic conditions many live with daily.
Given the positive outcomes observed, lanreotide’s application may soon represent more than just another treatment option; it could pave the way for refreshed guidelines and pathways for managing diabetic macular edema effectively. This treatment may become instrumental not only for lasting visual improvements but also for tackling broader metabolic challenges presented by diabetes, reflecting its utility across the clinical and therapeutic spectrum.