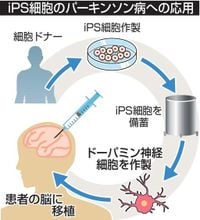
In a groundbreaking clinical trial, a research team from Kyoto University has made significant strides in treating Parkinson's disease using human induced pluripotent stem cells (iPS cells). This pioneering study revealed that among the six patients involved, four showed noticeable improvement in their symptoms, marking a historic moment in regenerative medicine.
The trial, which began in August 2018, involved patients aged between 50 and 69. The researchers transplanted between 5 million to 10 million iPS cell-derived nerve cells into the brains of the participants. The results were published in the British scientific journal Nature on April 16, 2025, and have generated considerable excitement within the medical community.
Parkinson's disease is a debilitating condition characterized by the gradual loss of dopamine-producing nerve cells in the brain, leading to symptoms such as tremors and impaired movement. Japan is home to approximately 290,000 individuals suffering from this challenging illness, which currently lacks a fundamental cure. Existing treatments primarily focus on supplementing dopamine through medication, but do not address the underlying loss of nerve cells.
Director Jun Takahashi of the Kyoto University iPS Cell Research Institute, who has dedicated over two decades to this field, expressed his optimism at a recent press conference. "I am happy that there are patients whose symptoms have improved, and I am grateful to the patients who underwent the treatment," he stated. This sentiment reflects the hope that iPS cell therapy could potentially transform the treatment landscape for Parkinson's disease.
The clinical trial confirmed that dopamine production was evident in the brains of all six patients, except for the first one, whose safety was only verified. Notably, those who received a higher number of transplanted cells exhibited greater dopamine production. Importantly, no serious adverse effects, such as the cells becoming cancerous, were reported.
Furthermore, when comparing the motor functions of patients before and after the transplantation, four of them showed improvements even without taking dopamine-supplementing medication. When medication was used in conjunction, five patients experienced symptom relief. Interestingly, younger patients with less advanced symptoms tended to respond better to the treatment.
Despite the promising results, a survey conducted among patients regarding their quality of life before and after the transplantation revealed no significant changes. Associate Professor Ryosuke Takahashi, who served as the principal investigator, acknowledged this as a future challenge, stating, "Future issues to be addressed include working to improve this aspect."
Looking ahead, Sumitomo Pharma, the pharmaceutical company involved in the trial, plans to apply for manufacturing and sales approval from the government for this regenerative medicine product. Takahashi expressed his ambition, saying, "We aim to obtain approval within the year 2025." This step could pave the way for the first-ever cell therapy using iPS cells to be available for clinical use.
The significance of this trial cannot be overstated. It not only demonstrates the safety and efficacy of iPS cell-derived therapies but also positions Japan as a leader in the global race for advanced regenerative medicine technologies. The results underscore the potential of iPS cells, which are capable of developing into various cell types, to revolutionize treatment options for currently incurable diseases.
As the field of regenerative medicine continues to evolve, the Kyoto University team's findings are expected to spark further research and development efforts both domestically and internationally. Other universities and companies around the world are also exploring the potential of iPS cells, intensifying the competitive landscape for breakthroughs in this area.
To realize the full potential of iPS cell therapies, establishing a mass production system and reducing manufacturing costs will be crucial. As the technology matures, the hope is that these therapies will become widely accessible to patients in need.
In conclusion, the successful outcomes of this clinical trial represent a pivotal moment in the quest for effective treatments for Parkinson's disease. With the potential for regulatory approval on the horizon, the research team's dedication and the patients' willingness to participate in the trial have laid the groundwork for a promising future in regenerative medicine.