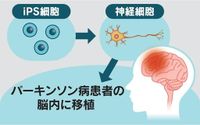
On April 17, 2025, the Kyoto University iPS Cell Research Institute (CiRA) announced promising results from a clinical trial focusing on the treatment of Parkinson's disease using induced pluripotent stem (iPS) cells. This groundbreaking study revealed that symptoms improved in four out of six patients who received nerve cells derived from iPS cells, marking a significant step towards what could be the world's first practical application of this innovative therapy.
Parkinson's disease, a neurodegenerative disorder that affects approximately 290,000 people in Japan alone, is characterized by the degeneration of dopamine-producing neurons in the brain. This leads to severe motor function impairments, including tremors and rigidity. Currently, treatment options primarily focus on alleviating symptoms rather than addressing the underlying causes of the disease.
The clinical trial, conducted at Kyoto University Hospital from 2018 to 2023, involved the transplantation of five to ten million dopamine nerve cells into the central part of the brain of seven patients aged between 50 and 69. Over the course of two years, the researchers monitored the patients to assess both the effectiveness and safety of the treatment. Remarkably, no serious side effects were reported among the participants, and in the six patients evaluated for efficacy, all showed increased dopamine activity in the brain following the transplantation.
Professor Jun Takahashi, the director of CiRA, expressed his enthusiasm about the results, stating, "It is a great achievement that the therapeutic effect has been confirmed in patients. We want to deliver cell transplantation treatment to as many patients as possible as soon as possible." This optimism is echoed by the pharmaceutical company Sumitomo Pharma, which supplied the iPS cells for the trial and is preparing to apply for approval of a regenerative medicine product. The company hopes to secure this approval within the current fiscal year.
The research findings were published in the prestigious British journal Nature on April 16, 2025, highlighting the significance of these developments in the field of regenerative medicine. The study's success not only showcases the potential of iPS cells in treating Parkinson's but also opens avenues for future research into other neurodegenerative diseases.
In the context of global health, Parkinson's disease affects around 10 million individuals worldwide, with no definitive cure currently available. The causes of the disease remain largely unknown, and it is classified as a difficult-to-treat condition by health authorities. The current treatment landscape primarily involves symptomatic therapies, which do not prevent disease progression.
As the clinical trial results illustrate, the use of iPS cells may lead to a paradigm shift in how Parkinson's disease is treated. By replacing lost or damaged neurons, this approach aims not only to improve motor functions but also to address the root causes of the disease. The implications of such advancements could be profound, potentially restoring quality of life for countless individuals suffering from this debilitating condition.
Looking ahead, Sumitomo Pharma is expected to submit its application for manufacturing and sales approval to the Ministry of Health, Labor and Welfare as early as this summer. If successful, this would mark a significant milestone in the commercialization of iPS cell-derived therapies, with Sumitomo Pharma potentially becoming a pioneer in this emerging field.
Moreover, the success of this clinical trial could pave the way for additional research initiatives and collaborations aimed at harnessing the power of iPS cells to combat other neurological disorders. For instance, the recent application for approval by KoaLプス, an Osaka University startup, for a myocardial sheet designed for severe heart disease, underscores the growing interest in iPS cell technology across various medical fields.
Professor Takahashi remains hopeful about the future, stating, "While the confirmation of therapeutic effects is a significant achievement, it is merely a stepping stone. We are committed to advancing our research to provide better treatments for patients." This sentiment resonates with many in the scientific community, who view this as a crucial moment in the journey towards effective therapies for neurodegenerative diseases.
The results of this trial not only bring hope to patients and their families but also exemplify the potential of cutting-edge scientific research in transforming healthcare. As the world watches closely, the developments surrounding iPS cell therapies could redefine the landscape of treatment options for Parkinson's disease and beyond.