A critical study highlights the role of serine 225 phosphorylation in the hepatitis C virus (HCV) non-structural protein NS5A, revealing it as a key regulatory event for viral replication and assembly. Researchers have demonstrated that S225 phosphorylation influences the localization and interactions of NS5A, with implications for treatment strategies with direct-acting antivirals (DAAs) like daclatasvir.
The investigation into NS5A, a multifunctional protein essential for HCV life cycle, reveals that S225 is crucial for maintaining the protein’s integrity and function. Hepatitis C, affecting an estimated 58 million people globally, leads to serious health issues such as chronic liver disease and hepatocellular carcinoma. Understanding the molecular intricacies of HCV is vital for developing effective treatments.
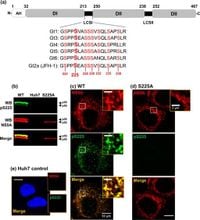
Utilizing a newly developed antiserum that specifically targets pS225, the study employed advanced imaging techniques such as super-resolution microscopy. These methodologies provided insights into the spatial organization of NS5A. The data indicate that phosphorylated NS5A exists predominantly in hyper-phosphorylated states, which are essential for the protein’s correct localization within the cell.
The presence of pS225 near lipid droplets within HCV-infected cells suggests a connection between viral replication processes and cellular lipid metabolism. This architecture of punctae formed by NS5A is disrupted when treated with daclatasvir, indicating that the drug affects not only replication but also the structural dynamics of viral assembly.
Furthermore, the research proposes that S225 phosphorylation primes multiple sequential phosphorylation events, enhancing our understanding of the regulatory mechanisms underlying NS5A's multifaceted roles. This modification is especially significant considering the challenges faced in HCV treatment, where current therapies target viral proteins like NS5A but often bypass the mechanistic understanding of their actions.
The findings highlight that while daclatasvir efficiently inhibits HCV replication, it also induces observable changes in the physical architecture of NS5A-positive clusters, reinforcing the importance of understanding both the biochemical interactions and the broader implications of such architectural alterations in HCV biology.
By shedding light on the functional relevance of S225 phosphorylation, this study opens new avenues for developing more effective HCV therapies, showcasing the potential benefits of targeting specific phosphorylation events within viral proteins. The conservation of this phosphorylation site across various HCV genotypes may also indicate a broader role in viral biology that warrants further exploration.
As researchers continue to decode the complexities of HCV and its interactions with host cells, studies like this will underpin future advancements in antiviral strategies, ultimately aiming to improve clinical outcomes for millions of affected individuals worldwide.