The increasing age of populations worldwide is leading to significant public health challenges, one of which is the rising incidence of pancreatitis among the elderly. An intriguing new study sheds light on how cellular changes, particularly within pancreatic β-cells, contribute to this troubling trend. Researchers have discovered a specific microRNA, miR-503-322, secreted by senescent β-cells, which plays a pivotal role in driving both chronic and acute pancreatitis by targeting MKNK1, a protein integral to the function of acinar cells within the pancreas.
Aging is recognized as the primary risk factor for chronic pancreatitis, a condition often characterized by recurrent episodes of abdominal pain and the potential for severe complications. The connection between chronic inflammation, aging, and the pancreas is complex and not entirely understood. The study published by researchers from Tianjin First Central Hospital and Nanjing Medical University reveals new mechanistic insights addressing this significant health issue.
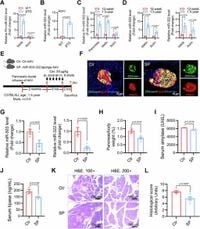
Throughout the study, the researchers explored the hypothesis wherein senescent β-cells contribute to the onset of pancreatitis through the elevation of miR-503-322, which is released as small extracellular vesicles. These vesicles enter acinar cells—the cells responsible for secreting digestive enzymes leading to autodigestion if dysregulated. The team employed genetically modified mouse models (specifically βTG mice and miR-503-322 knockout models) to assess the dynamics between miR-503-322 expression and acute pancreatitis (AP) severity.
Key findings show the secretion of miR-503-322 increases with the physiological aging of β-cells, leading to inhibited secretion of necessary digestive enzymes by acinar cells. The research highlighted, "These findings highlight the miR-503-322–MKNK1 axis mediates the endocrine-exocrine regulatory pathway, especially in aged mice and humans." This direct relationship suggests the elevated microRNA not only affects the secretion of digestive enzymes but also contributes to tissue repair and regeneration failures following injury.
Mechanistically, miR-503-322 targets MKNK1, inhibiting acinar-cell secretion and promoting deleterious effects, such as autodigestion of pancreatic tissue. Notably, the study observed higher levels of miR-503-322 correlated negatively with serum levels of amylase, another enzyme necessary for proper digestion, which suggests the presence of pancreatitis.
To establish causation, the researchers conducted various experiments, including inducing acute pancreatitis with caerulein, which revealed enhanced severity and pathological changes such as pancreatic edema and significant macrophage infiltration in mice with elevated miR-503-322. They observed severe pancreatitis-like changes and significant exacerbation of caerulein-induced acute attacks correlated with chronic pancreatitis, indicating the substantial impact elevated miR-503-322 has on the pancreas during aging.
Conversely, ablation of miR-503-322 demonstrated significantly ameliorated outcomes concerning caerulein-induced acute pancreatitis. The team reported, "Blocking miR-503-322 in β-cells of aged mice showed good inhibitory effects on pancreatitis," underlining potential therapeutic strategies for intervention.
These compelling findings provide insights not only suitable for advancing our scientific knowledge of pancreatitis's etiology among the elderly but also suggest new avenues for treatment. Possible therapeutic strategies might involve modulating the miR-503-322 axis to restore normal pancreatic function.
The research's focus on aging-associated diseases unravels the interplay between different types of pancreatic cells and challenges the traditional anatomical barriers, illuminating rich avenues for future investigations. Targeting miR-503-322 could serve as promising preventive and therapeutic strategies for mitigating the risks of pancreatitis, especially among the elderly who struggle with this severe and often debilitating condition. Whether similar mechanisms apply to other age-related diseases remains to be investigated, but the prospects of such research could advance personalized medicine and treatment approaches.
Overall, this comprehensive research not only enriches our knowledge around the influence of senescent cellular behavior on pancreatitis but also piques interest for additional studies aimed at clarifying the role of microRNAs as mediators of age-related disease.