In a groundbreaking study published in 2025, researchers at the University of California, San Francisco, have unveiled critical insights into the development of microglia, the brain's resident immune cells. By examining the mechanisms by which microglia mature, the team identified integrin beta 8 (Itgb8) as a pivotal factor that activates transforming growth factor beta 1 (TGFβ1), playing a crucial role in microglial development.
Microglia are essential for brain function and health, serving as immune sentinels that respond to injury and maintain neural homeostasis. The study highlights the significance of the interplay between genetic programming and environmental signals in shaping microglial diversity. The findings illuminate how perturbations in these processes can lead to dysfunction, with wide-ranging implications for neurodevelopment and disorders.
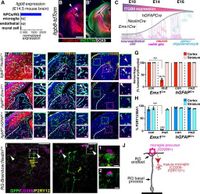
The researchers employed conditional knockouts in mice to investigate the signaling pathways involving Itgb8 and TGFβ1. By deleting Itgb8 from radial glia progenitors—cells critical for brain development—they observed that microglia became developmentally arrested and displayed a dysmature phenotype. The absence of Itgb8 led to a lack of TGFβ1 activation, which in turn resulted in severe neuromotor symptoms closely mimicking those seen in Itgb8 mutant mice. "In the absence of autocrine TGFβ1 signaling, microglia adopt a similar phenotype, leading to neuromotor symptoms almost identical to Itgb8 mutant mice," wrote the authors of the article.
These findings illustrate the necessity of TGFβ signaling for microglial maturation. Previous studies have shown a correlation between abnormal microglial development and various neurodegenerative diseases; however, the mechanisms driving these relationships have been poorly understood. This study addresses these gaps, shedding light on the spatio-temporal regulation of TGFβ signaling essential for microglial development and function. "This study describes the spatio-temporal regulation of TGFβ activation and signaling in the brain necessary to promote microglial development," the authors noted.
By utilizing various experimental techniques, including transcriptional and epigenetic analyses, the researchers demonstrated how Itgb8 expression in radial glia influences microglial differentiation. Their work suggests that dysmature microglia lack the capacity to respond adequately to environmental cues, which may drive neuroinflammation and other pathological states in the nervous system.
The implications of these findings extend beyond developmental biology. They suggest novel therapeutic targets for neurodegenerative and neuroinflammatory diseases, which are often associated with microglial dysfunction. By understanding the molecular pathways underlying microglial maturation, scientists can develop strategies to manipulate these processes, potentially offering new avenues for treatment.
In summary, this study provides a comprehensive exploration of the systems controlling microglial development, emphasizing the importance of Itgb8-mediated TGFβ1 activation. As our understanding of microglial biology deepens, so too does our capacity to address the myriad forms of neural dysfunction that result from their dysregulation.
With ongoing research aimed at elucidating the detailed pathways involved in these processes, the future holds promise for innovative therapies targeted at restoring normal microglial function, paving the way for improved outcomes in conditions linked to disturbed microglial activity.