New research highlights the intricate workings of LYVE-1, a critical receptor that facilitates immune cell entry into lymphatic vessels, shedding light on its distinctive interaction with hyaluronan (HA). This study reveals an innovative sliding mechanism that allows immune cells to traverse endothelial junctions with ease, a finding that could significantly impact our understanding of immune responses in health and disease.
LYVE-1, or LYmphatic Vessel Endothelial receptor-1, has long been recognized for its role in helping immune cells migrate through lymphatic vessels. This migration is essential for maintaining immune surveillance and instigating appropriate immune responses. However, until recently, the precise mechanics of how LYVE-1 interacts with HA, a key glycosaminoglycan found on the surface of immune cells, had remained elusive.
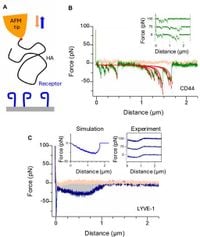
The current study employed a combination of dynamic force spectroscopy (DFS) and X-ray crystallography to uncover the binding dynamics of murine and human LYVE-1 complexes. The research illuminates the unique sliding interaction LYVE-1 exhibits with HA, allowing immune cells to transition from tissues into lymphatic capillaries with reduced friction. "These findings explain the mode of action of a dedicated lymphatic entry receptor and define a distinct, low tack adhesive interaction that enables migrating immune cells to slide through endothelial junctions with minimal resistance," wrote the authors of the article.
Until now, the process by which LYVE-1 binds to HA and facilitates cell migration had not been clearly understood. The new insights emphasize the importance of the non-reducing ends of the HA chain, which LYVE-1 preferentially engages. This preference enhances the receptor's functionality in lymphatic entry, as identified during the experiments that revealed the sliding behavior.
In contrast to LYVE-1, the closely related receptor CD44 operates through a mechanism characterized by individual bond ruptures when binding to HA, a significantly different process compared to the cooperative nature of LYVE-1's interaction. The researchers noted, "The capacity of the HA chain to first displace and then form dynamic H bonds with the surface water layer is consistent with its function as a lubricating cushion around the sugar." This illustrates how LYVE-1 promotes efficient migration across lymphatic endothelium.
The implications of these findings extend beyond basic biological understanding—they could pave the way for innovative therapeutic strategies targeting immune cell trafficking. Enhancing or inhibiting LYVE-1 functionality may influence how immune responses are regulated in various diseases, from infections to cancer.
Importantly, the study also uncovered the intricate co-dependence of LYVE-1 and HA in facilitating immune cell movement. LYVE-1 binds with a significant reliance on the structural integrity of the HA chain, suggesting that manipulating HA availability could alter immune cell positioning in tissues, thereby modulating the immune response. As the authors conclude, ongoing studies should elucidate how these distinct properties of LYVE-1 can be exploited for the development of interventions aimed at modulating immune function.
As our understanding of immune mechanisms advances, LYVE-1 stands out as a promising candidate for targeted therapeutic developments. Understanding its unique binding properties and the mechanics of its interactions require researchers to further explore the receptor's roles in both normal physiology and pathological conditions. By doing so, scientists can leverage these insights for strategic advancements in immune therapies.
This study not only fills a critical gap in our understanding of immune cell migration but also highlights a distinct functional mechanism within the broader complexities of immune responses.