Researchers at Westlake University have developed an innovative molecular framework known as StimExo, a breakthrough technology that allows for user-defined control signals to mediate on-demand calcium-dependent exocytosis. This significant advance promises to transform cell therapy by enabling immediate protein secretion, particularly advantageous in treatments that require rapid responses, such as in diabetic patients.
Cell-based therapies have gained traction due to their potential to program human cells to react to disease or other stimuli. Traditional strategies for managing conditions like insulin resistance and type-2 diabetes rely on slower transcriptional control mechanisms, lacking the speed required for acute treatments. StimExo circumvents these limitations by harnessing sophisticated synthetic biology techniques to allow for instantaneous secretion of therapeutic proteins in response to specific environmental cues.
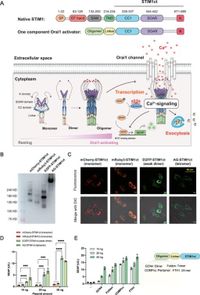
The StimExo technology integrates a modular framework that incorporates engineered bipartite activators of calcium release-activated calcium (CRAC) channels, facilitating calcium influx that is flexibly regulated by various small molecule triggers. In tests with diabetic mice, the authors successfully demonstrated how this framework can be utilized to induce rapid insulin secretion in response to the FDA-approved drug grazoprevir. "This work achieves true 'sense-and-respond' cell-based therapies and provides a platform for remote control of in vivo transgene activities using various trigger signals of interest," wrote the authors of the article.
The newly engineered system enables drug-responsive protein secretion at impressive speeds. Previously, similar regulatory mechanisms primarily depended on deploying temperature, light, or voltage-sensitive channels, which presented challenges in clinical applicability. According to the researchers, StimExo simplifies this complexity by attaching known human-derived proteins, mitigating compatibility and safety concerns often associated with foreign components.
Upon administration of grazoprevir, insulin-secreting pancreatic cells engineered with StimExo responded within minutes by elevating intracellular calcium levels. This rapid response time is vital for managing blood glucose levels in diabetic conditions, where immediate insulin delivery can make a life-saving difference. In fact, real-time experiments showed that gene delivery, coupled with this novel exocytosis technology, helped control blood glucose homeostasis in the diabetic mouse model.
As detailed in their findings, the method allows for instancing therapeutic payloads from various human endocrine cell types within a few minutes after stimulation, marking a significant progress in drug-delivery systems outside conventional practices. Taking this work further, scientists are optimistic about applying StimExo technology to other complex physiological processes where precise timing and dosage are crucial.
During the experimental trials, researchers encapsulated the engineered pancreatic cells into biocompatible alginate-poly-L-lysine beads, establishing a method for protecting cell integrity while allowing for dynamic protein secretion. An essential aspect of this approach is ensuring that the system can be scaled effectively for broader applications. "StimExo overcomes a critical hurdle that was hindering instant customizable therapies," wrote the authors of the article.
The studies showcase how StimExo can significantly streamline the process of protein secretion, hence redefining the boundaries of therapeutic interventions for diseases that demand immediate responses. With its potential to influence various therapeutic areas, from diabetes to other metabolic disorders requiring tailored treatments, there is a pronounced impact expected from future research employing these principles.
The implications of this research go beyond diabetes management alone. By switching the use of pharmaceuticals that regulate transgene expression with antivirals like grazoprevir, researchers can establish safer, more effective strategies. This paradigm shift emphasizes the adaptability and user-defined nature of StimExo, promising a new era of personalized medicine where patient feedback directly informs drug administration, minimizing the risks traditionally associated with therapeutic interventions.
In conclusion, the StimExo platform differentiates itself through its versatility and rapid response capabilities, marking it as an asset for future gene therapies. While significant advancements have been made, ongoing research will focus on optimizing delivery methods and exploring further applications of this transformative technology in diverse medical settings.