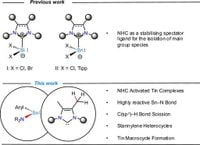
In a groundbreaking study published in Nature Communications on March 18, 2025, researchers have unveiled a new approach to activating C(sp3)–H bonds using N-heterocyclic carbenes (NHCs) and stannylenes. This work highlights the ability to manipulate the reactivity of these traditionally inert compounds, enabling the formation of new materials with potential applications in various fields.
N-heterocyclic carbenes have long been utilized in chemistry due to their incredible stabilizing effects on reactive compounds. However, they are often seen as passive participants in chemical reactions. This research challenges that perception by demonstrating how specific combinations of NHCs with stannylenes can lead to significant reactivity. The team, led by researchers from Georg-August-Universität Göttingen, discovered that pairing heteroleptic terphenyl-/amido-based stannylenes with tetra-alkyl substituted NHCs drastically changes the interaction dynamics, making the stannylenes both highly reactive and isolable.
At the core of the investigation was the reaction of a stannylene complex (1a) with an NHC known as IMe4 (1,3,4,5-tetramethylimidazol-2-ylidene). Conducting this reaction in benzene at room temperature yielded a stable NHC-ligated stannylene (2a) with a yield of 61%. X-ray crystallography confirmed the formation of 2a, revealing the stannylene had a Sn–CNHC bond length of 2.3255(9) Å, which falls within expected ranges for such complexes.
As the researchers heated the solution to 80 °C, they observed an effective transformation of 2a back into the stannylene and the release of hexamethyldisilazane (HN(SiMe3)2), indicative of the C(sp3)–H activation process. With each reaction producing these new products, the researchers demonstrated a high degree of control over the reactions enabled by the sterics of both the NHC and the coordinated ligands.
This breakthrough extends beyond just stannylenes. By manipulating steric and electronic factors in the coordination sphere of stannylene complexes, the researchers developed various macrocyclic structures. Notably, they synthesized a tetranuclear macrocycle (3a) through consecutive C(sp3)–H activations involving the methyl backbone of the NHC. This novel approach unlocked a range of steric configurations that highlighted the tunability of the new synthetic methods and the potential for creating complex molecular architectures.
Further expanding the study, DippTerSn{N(SiMe3)2} (1b) and TippTerSn{N(SiMe3)2} (1c) were also reacted with IMe4, successfully yielding a range of novel NHC-adducts (2b and 2c). These reactions were characterized through NMR, revealing shifts indicative of significant chemical changes.
Later attempts combined these stannylenes with IiPr2Me2, leading to the formation of a stannylene heterocycle (5a) after initial reactions at higher temperatures. The formation of 5a exhibited an Sn–CNHC bond length of 2.316(2) Å and showcased the unique properties of the intramolecular C(sp3)–H activation mechanism.
Explaining their findings, the authors of the article summarized: “This work explores the limits of chemical reactivity in N-heterocyclic carbene-stabilized stannylenes, broadening our understanding of main group chemistry and offering new strategies to generate reactive organic materials.”
The implications of this research extend into fields ranging from material science to organic synthesis. The ability to tune the reactivity of stannylenes via NHCs marks a significant step forward in both coordination chemistry and materials design. The manipulation of bond scissions to create metallocycles opens new avenues for synthesizing complex organic compounds, making this research pivotal for future explorations in modern chemistry.
Researchers plan to further explore the potential applications of these macrocyclic systems in template-controlled synthesis and molecular recognition, promising to bridge understanding between fundamental chemical interactions and practical uses in industrial contexts.