Researchers have recently introduced a groundbreaking approach to enhancing aqueous zinc metal batteries, which are considered a promising alternative in energy storage technology due to their cost-effectiveness, safety, and high energy density. The study, published on March 24, 2025, in Nature Communications, unveils a well-designed organic/inorganic dual-phase solid-electrolyte interphase (SEI) that facilitates highly ordered zinc electrodeposition from single-crystal units to polycrystalline stacking.
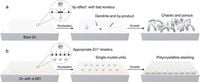
Aqueous zinc metal batteries (AZMBs) have gained attention for their low cost and high theoretical capacity. However, achieving a stable and durable zinc anode remains a significant hurdle due to issues such as hydrogen evolution reactions and irregular dendrite growth. These problems lead to structural instability and poor battery performance. Addressing these challenges, the researchers constructed a unique SEI that improves zinc electrodeposition processes, aiming for better stability and efficiency.
According to the authors, "the uncommon SEI with appropriate ion transport kinetics and thermodynamic stability protects deposited zinc from side reactions of hydrogen evolution and metal corrosion." This advancement enables rapid and controlled nucleation of zinc crystals, resulting in denser polycrystalline structures that significantly minimize the formation of dendrites, which are often responsible for battery failure.
The innovative dual-phase SEI was created using a specific electrolyte composition that includes zinc trifluoromethanesulfonate and tris(2-cyanoethyl)phosphine. This combination was found to homogenize zinc ion transport and stabilize the growth conditions for the zinc crystals. The researchers reported that symmetric zinc batteries utilizing this new SEI demonstrated a lifespan of over 5600 hours and a high depth of discharge of 85.0%.
Despite the challenges that have historically limited the efficiency of zinc batteries, this new approach is positioned to make significant contributions to battery technology. The superior stability and longevity were evidenced by the full cells testing, which resulted in a capacity retention of 201.9 mAh g−1 at high operational temperatures of -30°C. In addition, the 0.1 Ah Zn | |I2 pouch cell operated stably for 113 cycles, showcasing a high specific energy of 122.1 Wh kg−1, exemplifying the practical potential of this battery system.
To further investigate the properties of the dual-phase SEI, the researchers employed various analytical techniques, including differential electrochemical mass spectrometry (DEMS) and scanning electron microscopy (SEM). These examinations revealed that the SEI effectively curbs parasitic reactions while providing a conducive environment for crystal growth, ultimately enhancing the efficiency of zinc deposition processes.
X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) analyses confirmed the composition and structure of the SEI, revealing a well-organized interface that plays a critical role in regulating zinc ion flux and mitigating reaction by-products.
The improvements offered by the SEI extend to practical applications, with the potential for long-cycle life and higher capacity retention observed across various battery configurations. In tests, the Zn | |MnO2 batteries exhibited a high discharge capacity of 181.9 mAh g−1 after 150 cycles at a moderate current, while the Zn | |NaV3O8·1.5HO batteries maintained a stable discharge capacity over 8500 cycles.
These findings not only advance the understanding of the critical roles of SEI on zinc electrodeposition behaviors but also provide valuable insights into future directions for improving battery performance across different metal batteries. The experimental data underscores the importance of engineering specific interfacial structures to realize dense and orderly nucleation of metal deposits.
The implications of this research are profound, particularly as the need for sustainable and reliable energy storage solutions continues to grow. The researchers concluded optimistically, stating that "our findings advance the understanding of critical roles of SEI on zinc electrodeposition behaviors and provide valuable insights into crystal structure regulation during electrodeposition in other metal batteries.”