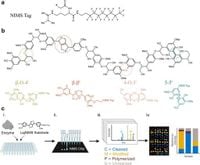
In a significant advancement for biomass research, a new study has developed a high-throughput platform to efficiently screen lignin-modifying enzymes (LMEs), which play a pivotal role in breaking down lignin - a complex polymer that constitutes a major component of renewable biomass. Utilizing an innovative nanostructure-initiator mass spectrometry (NIMS) technique, researchers can now characterize the activities of these enzymes on various model lignin compounds. The study, published on March 25, 2025, addresses the challenges of lignin valorization, where currently only 2% of lignin is utilized in industries despite its abundant availability as a renewable resource.
The researchers, including J.R. Onley, K. Gupta, and their colleagues, investigated four key lignin bond types - β-O-4’, β-β’, 5–5’, and 4-O-5’ linkages - using tagged model compounds designed to limit undesired polymerization during the testing process. By employing a robotics method to print tiny sample droplets on NIMS chips, the team effectively quantified enzyme activity across a range of pH levels from 3 to 10, ultimately discovering that the pH optimum varied based on the specific enzyme and substrate used.
"All tested enzymes oxidized the four substrates and cleaved the β-O-4’ and β-β’ substrates to monomeric products," stated the authors of the article. This breakthrough allows for more efficient and targeted enzyme application in converting lignin into valuable biofuels and bioproducts. As the demand for renewable energy sources grows, enhancing the use of lignin for sustainable processes presents a promising avenue for research and commercial applications.
The team performed over 2,000 reactions to characterize ten enzymes under different pH conditions, revealing that the horseradish peroxidases cleaved both β-β’ and β-O-4’ bond types across a broader pH range compared to laccases. This difference is significant: while the laccases displayed pH optima correlating with previous research, the horseradish peroxidases demonstrated remarkable adaptability. This finding supports the idea that enzymatic processes for lignin degradation need substrates specifically tailored for effective industrial application.
The implications extend into the realm of environmental sustainability. Current lignin processing methods are limited by harsh treatment conditions and yield poor recyclability. The new methods explored in this study could lead to greener, more efficient alternatives that do not fall prey to the drawbacks seen with traditional approaches. Enzymatic depolymerization not only minimizes environmental impact but also expands potential applications in biorefineries.
Interestingly, the research highlighted how pH affects bond cleavage mechanisms differently across various bond types. The findings suggest that different lignin feedstocks and pretreatment methods might necessitate tailored enzymatic solutions for optimal performance. As the authors noted, "the active pH range depended on both the substrate and the enzyme type," which indicates the nuanced relationships that exist in enzymatic reactions.
Additionally, the study piqued interest in further investigations into how these mechanisms might apply in real-world lignin sources, which differ significantly from model compounds. Future work aims to bridge this gap, potentially focusing on more complex lignins in native conditions, which may yield even more substantive findings about enzyme dynamics.
Overall, this pioneering research paves the way for innovative approaches in biomass conversion technologies. By shedding light on the behaviors of LMEs in various pH environments, it could catalyze more efficient strategies in biofuel production, aligning with global efforts toward sustainability and renewable resource utilization.