Recent advances in regenerative medicine have unveiled a novel hydrogel designed to enhance cartilage healing. The newly engineered hydrogel, known as HA-TP, exhibits dynamic properties crucial for the formation of cartilage organoids. This discovery, published in a recent article, proposes that the mechanical and biochemical environments can significantly influence tissue regeneration.
Articular cartilage injuries are common, particularly in athletes and aging populations, yet effective treatments remain challenging. Traditional approaches often involve invasive procedures or fail to regenerate the cartilage effectively. However, researchers have highlighted that the ultradynamic HA-TP hydrogel can mimic the natural extracellular matrix (ECM), creating conditions conducive to cellular growth and tissue repair.
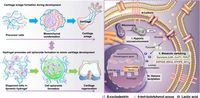
The study reveals that the HA-TP hydrogel allows for rapid mesenchymal condensation—an early phase essential for cartilage development—by creating a hypoxic microenvironment that enhances metabolic shifts necessary for cartilage formation. This finding opens new avenues for improving treatments for cartilage injuries, which often lead to debilitating conditions like osteoarthritis.
Using a novel approach, researchers encapsulated human mesenchymal stem cells (hMSCs) within the HA-TP hydrogel, showcasing its ability to promote the proliferation and organization of these cells. The dynamic properties of the hydrogel were crucial; as the cells multiplied, the hydrogel adapted its structure to accommodate the changes, establishing an optimal environment for cell aggregation and growth.
This innovative material was validated in both in vitro and in vivo models. In laboratory conditions, hMSCs encapsulated in the HA-TP hydrogel developed into large multicellular organoids, demonstrating advanced chondrogenesis—an essential requirement for effective cartilage repair. Notably, the organoids increased lactate production, a byproduct of glycolysis, furthering the cell’s differentiation towards cartilage formation.
In animal models, those implanted with the HA-TP hydrogel exhibited significantly improved cartilage repair compared to counterparts treated with conventional hydrogels. The results underscored the potential of this hydrogel in clinical settings, especially for treating extensive cartilage lesions.
Importantly, the study divulges the underlying mechanisms by which HA-TP exerts its effects. Enhanced lactylation—an epigenetic modification induced by lactate—was associated with improved expression of chondrogenic genes such as Sox9 and Aggrecan. This indicates a direct link between cellular metabolism and gene regulation during cartilage development, emphasizing the importance of metabolic cues in regenerative processes.
Furthermore, researchers found that the hydrogel's dynamic environment triggered histone lactylation on Lysine 18, which led to heightened transcriptional activity of essential cartilage regeneration genes. The novel approach of linking metabolic changes to epigenetic regulation presents a significant leap in understanding stem cell behavior in tissue regeneration.
As the potential of HA-TP hydrogel for clinical applications unfolds, the research community is optimistic about its implications for treating cartilage injuries. This material not only offers a solution for cartilage repair but also provides insights into the intricate relationship between metabolism and cellular behavior, paving the way for future innovations in tissue engineering.
The researchers emphasize that while their findings are promising, more extensive studies and clinical trials will be essential to translate these results into practice. The development of effective, less invasive methods for treating cartilage injuries could revolutionize current approaches in orthopedic medicine, ultimately improving the quality of life for countless patients.