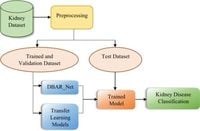
The field of nephrology takes a significant leap forward with the introduction of an innovative model called DBAR-Net, designed to automate the diagnosis and classification of common kidney diseases such as cysts, stones, and tumors.
This cutting-edge model employs advanced techniques in computer-aided design to transform how nephrologists tackle kidney diseases, seeking to improve clinical workflows while ensuring accurate results and efficient handling of large datasets. The DBAR-Net model incorporates a notable method known as multi-feature fusion, which addresses the challenges posed by complex and overlapping adaptive features seen in medical imaging.
Integral to the DBAR-Net methodology are the dual-fold convolved layer normalization blocks, referred to by their technical identifiers as CLN_b1 and CLN_b2. These components capture both fine-grained details and abstract patterns, ultimately contributing to faster convergence and improved robustness overall in model performance.
Two dedicated bottleneck attention modules, known as A_bam1 and A_bam2, further refine and enhance the convolved features. This refined processing enables a more focused representation of these features, directing attention to critical spatial regions. Additionally, the model employs dilated convolved layer normalization (DCLN_b), which enriches the contextual insights obtained from the semantic feature interpretation of visual data.
A key evaluation of the DBAR-Net model utilized the CT KIDNEY DATASET, a comprehensive collection encompassing 8750 CT images, which have been classified into four categories: normal, cyst, tumor, and stone. The experimental results from this dataset revealed remarkable findings, showcasing that the DBAR-Net model achieved a classification accuracy of 98.86% and an impressive F1 score of 0.98, positioning it as a formidable alternative to existing transfer learning models such as VGG16 and ResNet50.
The application of the DBAR-Net model carries significant implications for the field of kidney disease diagnosis. Traditional diagnostic methods, often reliant on manual examination of CT or PET scan images by nephrologists, can be time-consuming and prone to error. However, with the introduction of models like DBAR-Net, which automate the classification tasks through deep learning techniques, the timely detection and accurate categorization of renal diseases becomes feasible.
According to the authors of the study, "the model elevates integration and stability through the utilization of custom dual-fold convolved layer normalization blocks." It effectively addresses the internal discrepancies often found in datasets to minimize covariate shifts and enhance generalization.
Furthermore, the research team notes the potential for further enhancements within the DBAR-Net architecture. "Future research could focus on refining DBAR_Net architecture with advanced CNN features, integrating multi-modal data and optimizing transfer learning strategies for improved renal disease classification accuracy and clinical applicability," they wrote, indicating their commitment to innovation in this essential area of health.
The implications of this research extend beyond the laboratory and have the potential to shift clinical practices in nephrology significantly. By improving the accuracy and reliability of kidney disease detection at an early stage, the DBAR-Net model promises to contribute to better outcomes for patients facing renal health challenges.
As health professionals seek ways to improve patient care, technological advancements such as the DBAR-Net model stand as an example of the intersection of medicine and artificial intelligence. This model addresses not only the diagnostic implications but also highlights the ongoing journey towards more tailored and efficient healthcare delivery.