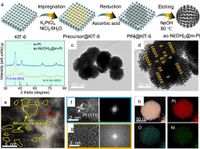
A new study has introduced an innovative catalyst that could significantly enhance the efficiency of hydrogen fuel cells by improving the process of oxygen reduction reactions (ORR). This catalyst, known as ac-Ni(OH)2@m-Pt, is characterized by its unique amorphous-crystalline heterostructure, consisting of nickel hydroxides interfaced with platinum, enabling enriched electron interactions that boost performance and stability.
Fuel cells, particularly proton-exchange membrane fuel cells (PEMFCs), represent a significant advancement in green energy technology, offering an alternative to fossil fuels. However, the sluggish kinetics of the ORR have been a persistent barrier to improving their efficiency. Traditional platinum-based electrocatalysts, while effective, are costly and often lack both mass activity and stability. As such, researchers are actively seeking strategies to reduce reliance on platinum without compromising performance.
An innovative approach proposed by the team led to the formation of a mesoporous heterojunction catalyst that combines the strengths of both amorphous and crystalline phases. The ac-Ni(OH)2@m-Pt not only possesses highly effective catalytic capabilities, achieving a remarkable mass activity (MA) of 0.95 A mgPt−1 but also maintains an impressive durability, with nearly 90% retention of its activity after 15,000 cycles of accelerated durability tests (ADTs).
The catalyst's performance benefits from the dynamic electron redistribution occurring at the a-c interface. This interaction facilitates quicker activation of oxygen, which is crucial for efficient catalysis. Specifically, the high performance was indicated by a half-wave potential of 0.91 V versus a reversible hydrogen electrode (RHE) with a four-electron selectivity of 98% in acidic conditions.
In addition, the ac-Ni(OH)2@m-Pt catalyst displays a high electrochemical surface area (ECSA) of 88.7 m2 g−1, which is substantially more than both traditional platinum catalysts and existing commercial counterparts.
“This work offers a promising avenue for improving the design of electrocatalysts using a-c interface engineering,” noted the authors of the article. The catalyst advances current understanding of the role an amorphous-crystalline interface can play in enhancing electrocatalytic reactions and provides a strategic model for future research.
The ac-Ni(OH)2@m-Pt catalyst was developed using a ‘template-solvent’ strategy that allows precise control over its structural characteristics. The study elucidated the dynamic cycling of electrons at the interface, where electrons are first captured from platinum to promote the activation of the catalyst and then returned, supporting the ORR process.
Testing results highlighted that ac-Ni(OH)2@m-Pt exhibited substantial advantages in both mass activity and stability. The catalyst not only performed exceptional electrochemistry but also retained its structural integrity through rigorous testing. Through advanced characterization techniques, including X-ray absorption fine structure (XAFS) analysis and synchrotron infrared (SRIR) spectroscopy, the researchers demonstrated that the coupled behaviors of amorphous and crystalline phases contribute to its efficacy during the ORR.
The study revealed that, during the reaction process, the amorphous phase could modulate the electronic structure to optimize adsorption energies, which in turn reduces the overpotential needed for efficient catalysis. The output not only supports the theoretical framework for future electrocatalyst design but also opens up pathways for real-world applications in hydrogen fuel cells.
Ultimately, while the research marks significant progress in catalyst construction, challenges remain regarding the scalability and long-term stability that will be critical as development moves towards commercialization. The findings in this study hold promise for achieving optimal performance in platinum-based catalysts through interface engineering. Future endeavors should focus on optimizing these catalysts further to meet industry standards for performance and durability.