Research on systemic infections caused by Candida albicans, an opportunistic fungal pathogen, highlights the importance of the immune response mediated by interleukin-1 (IL-1) receptors. A new study published demonstrates the pivotal role of IL-1 receptor (IL-1R) signaling, especially within non-hematopoietic cells, to combat severe candidiasis.
Systemic candidiasis occurs when the fungus breaches barriers and enters the bloodstream, posing significant dangers particularly to immunocompromised individuals. Such infections have alarmingly high mortality rates, estimated between 46-75%, underscoring the dire need for effective treatment strategies.
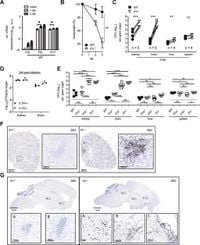
Despite the existence of antifungal medications, their effectiveness can be limited. The study, conducted using mouse models deficient in Il1r1, provides insights on how IL-1R is necessary for conferring resistance to infections by regulating immune cell recruitment and cellular metabolism.
By creating global and conditional knockout mice, the researchers isolated the effects of IL-1R signaling. Their findings revealed intriguing dynamics: IL-1R signaling primarily functions through non-hematopoietic cells, like those found in the kidney and brain, to elicit protective responses.
When the fungus Candida infects the body, it primarily targets organs such as the kidneys and the brain. Notably, kidney endothelial cells play a significant role, as their IL-1 receptors are involved in controlling fungal infections, independent of neutrophil recruitment, which is traditionally seen as the body's immediate first line of defense.
Interestingly, the study showed higher fungal counts and weight loss in Il1r1-deficient mice after infection, attributed to disrupted IL-1R signaling pathways, resulting in heightened metabolic activity and hypoxia. Indeed, the lack of IL-1R is linked to localized hypoxic conditions—which actually promote fungal growth and virulence—hindering effective immune responses.
“By acting on most non-hematopoietic cells of the kidney, IL-1R signaling suppresses metabolic responses to infection, protecting from local hypoxia which might otherwise facilitate fungal overgrowth,” wrote the authors of the article. This suggests not only the need for IL-1R signaling in combating infections but also hints at metabolic pathways’ roles during Candida infections.
The research utilized single-nucleus RNA sequencing which showed the shift to increased oxidative phosphorylation across various kidney cell types after infection. This metabolic switch highlights how cell behavior drastically changes during infection and the necessity of additional signaling pathways to suppress uncontrolled metabolic responses.
The study's outcomes indicate promising avenues for therapeutic developments. Understanding IL-1's broader impacts during Candida infections could pave the way for novel treatments aimed at reinforcing immune responses or inhibiting hyperactive metabolic shifts.
Effective combat against opportunistic pathogens like Candida albicans not only depends on immediate immune responses but also requires controlling the metabolic environment and sustaining cellular homeostasis.