A recently published study has spotlighted a previously unexamined link between the gut bacterium Cronobacter malonaticus and colorectal cancer, providing potentially groundbreaking insights into the role of gut microbiota in cancer progression. Researchers isolated a novel strain, designated PO3, from a fecal sample of a colon cancer patient and demonstrated its unique ability to promote cancer cell proliferation in both laboratory and animal models.
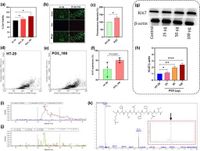
This investigation, led by a team at Yenepoya Medical College Hospital in Mangalore, India, involved rigorous analysis of the PO3 secretome—the substance secreted by bacterial cells—which exhibited significant effects on human colorectal adenocarcinoma cells. According to the study, the secretome from PO3 markedly increased cell viability and proliferation. Importantly, the research showed that the secretome enhanced fluorescence intensity levels and the expression of Ki-67, a known marker for cell proliferation, without triggering cell death. Such findings suggest the bacterium may have oncogenic properties.
Utilizing high-resolution mass spectrometry, the scientists identified a novel peptide, termed P506, within the PO3 secretome that exhibited strong stimulatory effects on colorectal cancer cell lines. Further experiments confirmed that synthetic versions of P506 also promoted a dose-dependent increase in proliferation among human colorectal adenocarcinoma cell lines.
In a series of in vivo experiments involving BALB/c mice, treatments with both the PO3 secretome and the synthetic peptide led to the development of colorectal polyps and histological changes indicative of malignancy. Mice treated with PO3 exhibited significant alterations in colonic architecture and dysplastic features compared to untreated controls. These findings highlight the potential risks associated with previously overlooked microbes in the gut, particularly those like Cronobacter malonaticus.
This research emerged from a meticulous process that included sampling feces from ten colon cancer patients who provided informed consent. The results suggest that beyond its known role in neonatal illnesses, Cronobacter malonaticus may be implicated in facilitating colorectal cancer, underlining the need for continued focus on the microbiome's influence on cancer.
The research team projects that further studies are needed to explore the specific mechanisms by which P506 and other microbial peptides could interact with host signaling pathways to influence cancer progression. This investigation not only broadens the understanding of colorectal cancer mechanism but also points towards innovative approaches in managing this prevalent condition by targeting gut microbiota.
In conclusion, this pivotal study underscores the importance of microbial-derived factors in cancer pathogenesis, advocating for further exploration into how such interactions might guide future therapeutic strategies against colonic malignancies.