Germany is taking a significant step to bolster global pandemic preparedness by moving to incorporate recent international health regulations into its national law. On Wednesday, July 16, 2025, the German Cabinet approved draft legislation designed to implement amendments to the World Health Organization’s (WHO) International Health Regulations (IHR), agreed upon by WHO member states in 2024. This move aims to enhance the global response to future health crises by improving the flow of critical information during outbreaks and ensuring sufficient laboratory and healthcare capacity worldwide.
Health Minister Nina Warken emphasized the urgency of the measure, stating, "The global community must be better prepared for global health crises." She cited the coronavirus pandemic as a stark lesson underscoring the need for stronger international cooperation and readiness. The updated regulations require countries to promptly report potential public health emergencies of international concern to the WHO, fostering transparency and rapid response coordination at a global scale.
Moreover, member states are expected to maintain "core capacities" in vital areas such as laboratory diagnostics and risk communication. These capacities are essential for accurately identifying and managing emerging infectious threats, thereby preventing their escalation into widespread pandemics. The draft legislation carefully balances international commitments with national sovereignty, as the Health Ministry reassured the public that the new rules would not compromise Germany’s ability to implement domestic protective measures.
Following the Cabinet’s approval, the draft law will be submitted to the German parliament for further deliberation and approval. If enacted, Germany will join a growing number of countries reinforcing their pandemic defenses by embedding WHO guidelines into their legal frameworks, a crucial step in strengthening global health security.
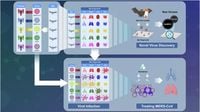
Meanwhile, groundbreaking scientific research is advancing our understanding of zoonotic viruses — those that jump from animals to humans — which are often the source of pandemics. A recent study published in the journal Science by Hyunjoon Kim and colleagues has unveiled the world’s most comprehensive bat organoid platform, marking a major breakthrough in comparative virology and pandemic preparedness research.
Bats are known reservoirs for numerous zoonotic pathogens, including coronaviruses, paramyxoviruses, and reoviruses. The research team developed organoids — miniature, lab-grown models of organs — representing four essential bat organ systems: airways, lungs, kidneys, and small intestine. These organoids were created using cells from five phylogenetically diverse bat species across Asia and Europe: Rhinolophus ferrumequinum, Myotis aurascens, Pipistrellus abramus, Eptesicus serotinus, and Hypsugo alaschanicus. These species are insectivorous bats with broad Eurasian distributions and are natural hosts to various zoonotic viruses.
The bat organoid platform allows scientists to systematically investigate virus-host tissue interactions under controlled laboratory conditions. By closely mimicking the cellular architecture and complexity of bat tissues, these organoids provide an unprecedented window into how viruses behave in their natural reservoirs. The platform also supports high-throughput antiviral drug screening, exemplified by findings that remdesivir, an antiviral drug, showed greater efficacy in bat organoids than in traditional cell lines, underscoring the translational potential of these models for therapeutic development.
One particularly intriguing discovery was the near absence of MUC5AC-positive goblet cells — mucus-producing cells — across all bat species studied. This suggests an evolutionary adaptation where bats minimize mucus production, possibly favoring enhanced airborne immune defenses. Such adaptations may contribute to bats’ remarkable ability to harbor viruses without suffering severe illness, a phenomenon that has long puzzled scientists.
The researchers tested infection patterns of major zoonotic pathogens including SARS-CoV-2, MERS-CoV, influenza A viruses, and Orthohantavirus Seoulense (SEOV) across the bat organoids. Infection patterns correlated with tissue-specific expression of viral entry receptors. For example, SARS-CoV-2 preferentially infected intestinal tissues of R. ferrumequinum despite the presence of ACE2 receptors in respiratory organoids, indicating that receptor presence alone does not guarantee successful infection. MERS-CoV showed broader tissue tropism, infecting lung organoids from multiple bat species, with infection efficiency linked to DPP4 receptor expression levels. Influenza A viruses exhibited strong respiratory tropism, facilitated by co-expression of both avian and human sialic acid receptors.
Importantly, the organoid models maintained functional innate immune responses. RNA sequencing revealed robust activation of interferon-stimulated genes (ISGs) following viral infection, with distinct response patterns depending on virus type and bat species. Notably, MERS-CoV triggered weaker immune responses compared to influenza A virus, suggesting differing viral strategies to evade host immunity.
The platform also enabled the isolation and characterization of previously unknown bat viruses from fecal samples, including mammalian orthoreovirus (MRV) and Shaanvirus-like paramyxovirus. These discoveries highlight the platform’s potential for virus discovery and surveillance, crucial for identifying emerging zoonotic threats before they spill over into human populations.
Beyond infectious disease research, bat organoids hold promise for aging studies. Bats exhibit exceptional longevity and healthy aging, with robust cancer resistance and efficient DNA repair mechanisms. Understanding these traits through organoid models could inform human aging research and strategies to promote healthy lifespan.
Despite these advances, the platform faces limitations. The lack of high-quality reference genomes for many bat species complicates genomic annotation and functional analyses. Additionally, current organoid models lack immune cell components, preventing comprehensive study of innate-adaptive immune interactions critical to viral pathogenesis. Future efforts aim to incorporate immune cells and expand organoid types to include endothelial, neuronal, and stromal tissues, broadening the platform’s applicability.
As the world grapples with the ongoing challenges of emerging infectious diseases, Germany’s legislative move to embed WHO’s International Health Regulations into national law aligns with scientific breakthroughs like the bat organoid platform. Together, these developments represent a multifaceted approach to pandemic preparedness — combining policy, surveillance, and cutting-edge research to safeguard global health.
Kim and colleagues’ study, published in Science (2025), exemplifies how innovative laboratory models can illuminate virus-host dynamics, facilitate antiviral drug development, and enhance our understanding of zoonotic spillover risks. Meanwhile, Germany’s commitment to strengthening international health regulations underscores the importance of coordinated global action to detect and respond to health threats swiftly and effectively.
Ultimately, the integration of scientific innovation with robust public health policy offers the best hope for preventing future pandemics and mitigating their impact on societies worldwide.