Retinoblastoma, a malignant tumor that primarily affects children, poses significant challenges in diagnosis and treatment, particularly when analyzing the genetic nuances associated with the disorder. Latest research sheds light on how variations in gene expression linked to anaplasia and laterality significantly impact patient outcomes.
In a comprehensive study published in March 2025, researchers delved into gene expression differences among retinoblastoma patients, specifically focusing on the effects of anaplasia—the abnormal growth of tumor cells—and laterality, whether the disease is unilateral (affecting one eye) or bilateral (affecting both eyes). Conducted by W. Li, K. Xu, and F. Chen, the study utilized two critical datasets—GSE141209 and GSE110811—from the Gene Expression Omnibus (GEO) database, involving tumor tissue data from 36 patients.
Retinoblastoma is the most common eye cancer in children, with incidence rates reported between 1 in 15,000 to 1 in 20,000 globally. The prognosis for patients can vary markedly based on whether the cancer is unilateral or bilateral, as well as the degree of anaplasia present. Unilateral cases generally have a better survival rate compared to bilateral cases, which often indicate more aggressive disease.
The researchers found significant differences in gene expression between patients categorized by anaplasia and laterality. As the authors noted, "the functions of synapse assembly and synaptic signaling were enhanced in clinical patients with severe anaplasia, while the function of photoreceptors was reduced.” Such findings suggest a crucial link between genetic expression profiles and clinical manifestations of the disease.
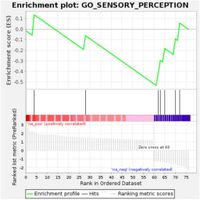
An analysis of the GSE141209 dataset revealed that among the 500 genes showcasing the most significant expression changes, 83 had P-values less than or equal to 0.05 and showed over a 1.5-fold change. These changes were notably enriched in sensory perception functions, underscoring the impact of anaplasia on visual-related gene expression. This suggests that certain sensory pathways are suppressed in patients with severe anaplasia.
In contrast, the GSE110811 dataset, which included a broader patient population, explored moderate anaplasia cases, revealing that 95 of the 1000 most significantly altered genes had similarly significant fold changes. The authors expanded upon these results, noting that there was a statistically significant functional enrichment related to abnormal eye conjugation movement and lipid metabolism in the unilateral group. This illuminates the complex genetic underpinnings that differentiate how the disease manifests in unilateral versus bilateral cases.
The data indicates that unilateral patients display enhanced expression of genes associated with eye movement and metabolic processes, while bilateral cases tended towards genes that inhibit cell proliferation. Such significant variations highlight how laterality affects genetic expression and clinical presentation.
Furthermore, the researchers observed that among unilateral patients, gene expression linked to sensory perception was significantly reduced in those with severe anaplasia. The findings suggest that retinoblastoma, particularly in severe cases, could exhibit a unique gene expression signature that may influence both prognosis and treatment decisions.
As the authors concluded, "Gene expression may be different in retinoblastoma patients with different anaplasias and lateralities.” This research not only enhances understanding of retinoblastoma's genetic landscape but may also have implications for targeted treatment strategies that consider an individual patient's genetic profile.
The implications of such findings underscore the necessity for further research to untangle the complexities of gene expression regulation in retinoblastoma. Understanding how these genetic differences play a role in disease progression could lead to more effective diagnostic and therapeutic approaches for this challenging pediatric cancer.
In light of the limited sample sizes inherent in some datasets, the need for larger studies to validate these initial findings remains crucial. Future research aspirations include exploring how genetic mutations, both germline and somatic, might regulate gene expression and influence clinical outcomes, ultimately improving survival rates for retinoblastoma patients.