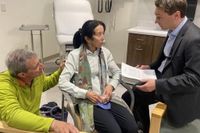
In a groundbreaking moment for Parkinson's disease treatment, a patient from the University of Colorado Anschutz Medical Campus became the first person in the nation to receive adaptive deep brain stimulation technology, marking a significant advancement in managing this progressive neurological disorder.
On March 21, 2025, Kate, a 75-year-old woman who has struggled with Parkinson's since 2019, experienced the new treatment firsthand. Reporting her emotions to reporters and healthcare staff, she stated, "It’s been a long time, and I have grandchildren. I really want to see my grandchildren, see them grow. And it’s given me hope." Kate’s enthusiasm echoed the aspirations of nearly 1 million individuals affected by Parkinson’s disease in the United States.
Deep brain stimulation has been utilized for over three decades but now involves utilizing the patient’s own brain signals through advanced electrode identifier (EI) technology combined with adaptive stimulation. This therapeutic system actively adjusts the electrical signals sent to the brain based on real-time data from individual brain activity. Dr. Drew Kern and Dr. John Thompson from the CU School of Medicine unveiled this technology, which had received U.S. Food and Drug Administration (FDA) approval in February 2025.
During the demonstration, Dr. Kern highlighted the immediate improvements in Kate's motor functions. "It’s hopeful that this will certainly reduce the number of fluctuations, the on-off time, and give that individual that constant level function that they're looking for," he commented. Such adaptability in treatment could be revolutionary, providing personalized responses to symptoms that vary throughout the day.
Dr. Kern expressed gratitude for the technological leap forward, saying, "To bring that today to something that's now commercially available that used to be just an idea is really kind of, when you sit back and think about it, very nostalgic. It’s – I never thought we'd really get here." This sentiment resonates deeply with patients, particularly those like Kate, who long for improvement in their daily lives.
The treatment requires surgical implantation of electrodes in the brain, alongside a device similar to a cardiac pacemaker that controls the stimulation. Medtronic, the company behind this technology, has integrated advanced software to enable real-time adaptations based on the unique brain signals of each patient. This evolution means that healthcare providers can optimize therapy without extensive trial-and-error adjustments typical of previous deep brain stimulation systems.
In a demonstration that attracted significant attention, Kate’s tremors visibly decreased within moments as the new system was activated, showcasing the therapy's promise. "To get Kate to be able to sit like she is right now would have taken me hours to get her to look like this, which we literally just did in a matter of minutes. That’s really surprising," said Dr. Kern, while expressing profound hope for a better quality of life for those battling Parkinson's.
Dr. John Thompson emphasized the innovative nature of the EI technology, suggesting it transforms the conventional stimulation model. “This technology has the potential to have important clinical impact in people with Parkinson’s disease," he said, highlighting how it could significantly enhance patient care.
Looking forward, both Kern and Thompson anticipate that this breakthrough will inspire a wave of new research into understanding the complexities of Parkinson’s disease. There are many unanswered questions about how this technology could improve treatment outcomes: how it works optimally during different activities or its effects on non-motor symptoms.
Research funding from the National Institutes of Health (NIH) played an essential role in advancing these developments. However, with ongoing discussions in federal circles regarding budget cuts, concerns about future research financing surfaced. Kern voiced apprehensions about preserving progress made in Parkinson’s research, highlighting that without continuous funding, the momentum could stall.
As of next week, starting March 24, 2025, similar technologies are set to launch at multiple U.S. centers, with four procedures already scheduled for that Monday. As this advanced treatment becomes more widely available, it is poised to change the lives of millions grappling with the challenges posed by Parkinson's.
With great anticipation surrounding further adaptations of this innovative approach, the medical community and patients alike are hopeful for what the future holds. This new technology not only offers a glimpse into improved treatment but also represents a significant step forward in understanding and managing Parkinson's disease, demonstrating that ongoing research and development can yield tangible benefits.
As Kate concluded her remarks, she was filled with enthusiasm, stating, "It gives me hope." In a world eager for advancements in the care of debilitating diseases, this hopeful sentiment encapsulates the promise held within the halls of scientific progress, particularly for those enduring the daily struggles of Parkinson's disease.