A troubling situation has arisen at Tom's of Maine, the popular natural personal care brand known for its commitment to environmentally-friendly products. The U.S. Food and Drug Administration (FDA) has reported significant violations at the company's manufacturing facility located in Sanford, Maine. A recent inspection uncovered the presence of harmful bacteria and other contamination concerns, sparking alarm among consumers who rely on Tom's for their toothpaste and other hygiene products.
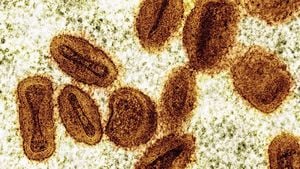
According to the FDA's findings, water used to manufacture Tom’s Simply White Clean Mint Paste was contaminated with the bacteria Pseudomonas aeruginosa. This organism can sometimes lead to serious illnesses, particularly infections of the blood and lungs, especially among individuals with compromised immune systems. This alarming discovery was part of multiple water samples taken at the facility between June 2021 and October 2022.
Further investigation revealed even more troubling issues, including the bacteria Ralstonia insidiosa, known for potentially causing severe infections such as sepsis, also found within the water supply of the manufacturing plant. Compounding the concern, Paracoccus yeei was detected within the finished product of Tom's Wicked Cool! Anticavity Toothpaste. This bacterium is notable for its potential resistance to antibiotics, posing additional risks should contamination occur.
The FDA’s warning letter, sent on November 5 to Colgate-Palmolive—parent company of Tom's of Maine—highlighted these significant violations. The letter detailed observations made during the inspection, including the presence of not only bacteria but also "a black mold-like substance" found near areas used for toothpaste production. The inspectors noted inadequate cleaning procedures and poor sanitary conditions at the facility. The FDA's findings came as part of a standard surveillance initiative, yet the severity of the violations discovered was far from routine.
Colgate-Palmolive's response to the FDA's inquiry has been deemed insufficient. The agency raised concerns about the company’s quality control measures, particularly around its water systems, noting failures to maintain adequate equipment cleanliness and overall facility hygiene. The FDA requested Tom’s of Maine to carry out remedial actions including developing comprehensive assessments of potential microbiological hazards and providing detailed cleaning protocols.
Despite Tom’s claiming regular testing of finished products, the FDA emphasized the importance of consistently evaluating the cleanliness of water used during manufacturing. The warning letter specifies the necessity of implementing microbial count limits to verify whether the water is suitably safe for such sensitive uses. The FDA also requested confirmation on whether any distributed products may have been affected by these contamination events.
Dr. Donald W. Schaffner, a food scientist, elaborated on the potential ramifications of the findings, expressing concern for customers, particularly those who might be immunocompromised. He stated, “If customers read about this issue and they have concerns, I think throwing out their toothpaste is a reasonable choice.” Such recommendations signal the seriousness with which experts are treating the current contamination crisis at Tom's of Maine.
After examining the various products and environments, the FDA has mandated Tom's to submit corrective plans addressing the contamination issues and detailing steps taken to prevent their recurrence. This entails re-evaluations of manufacturing designs, cleaning procedures, and the overall hygienic condition of the facility.
Caught up within the fallout of these findings is the trust and reputation built over decades by Tom’s of Maine. Founded on principles of sustainability and natural products, the brand has tapped deeply rooted values among consumers who prioritize health and environmental consciousness. The prospect of contamination and the swift action taken by regulatory bodies pose serious threats to this carefully cultivated public perception.
Tom’s of Maine has responded by stating their commitment to resolving the issues identified by the FDA, expressing faith in the safety of their products. They revealed plans to engage water specialists to fortify their sanitation processes as well as vowing to invest significantly in upgrades to the Sanford facility's water systems. Tom’s emphasized their adherence to quality control, maintaining their dedication to producing safe and effective products for their consumers.
At the moment, no formal recall has been issued concerning the impacted products. While Tom’s continues to work closely with the FDA, consumers are still encouraged to remain vigilant. It’s recommended for individuals who have purchased these affected products to thoroughly check their stock and stay informed of any updates from the company or regulatory bodies.
This incident serves as a reminder of the importance of rigorous health standards and regular inspections, especially within industries impacting public health. Consumers are becoming increasingly aware of the ingredients and sourcing behind their everyday products, leaning on transparency from brands as they navigate their choices. To have such revelations surface can be alarming but also highlights the diligence required at every stage of product development and manufacturing.
The incident at Tom's of Maine is still developing, and the ramifications are yet to be fully understood. Trust is fragile, and as Tom’s works to remedy the current failures, the onus lies on the company to rebuild the confidence of its loyal consumer base. With the federal oversight reminding everyone involved of the stakes, eyes will be trained on the company’s actions following this pivotal moment.