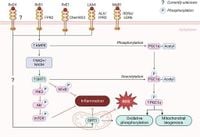
Alzheimer's disease (AD) remains the most prevalent form of dementia worldwide and represents one of the leading healthcare challenges globally. Its symptomatology is marked by progressive memory loss and a decline in cognitive functions, compounded by neurotoxic accumulation of amyloid-β plaques and pathological build-up of hyperphosphorylated tau. Economically, projections estimate that AD will incur costs of approximately $26.6 billion annually in Australia by 2041 and around $1.1 trillion annually in the United States by 2050, highlighting the urgency for effective preventative and therapeutic strategies.
Understanding the biological mechanisms underlying AD, despite being defined over a century ago, remains a complex and multifaceted challenge. Recent studies point to dysregulation in the phagocytic activity of microglia—an important immune cell type in the central nervous system—as a potential significant factor in the etiology of this neurodegenerative disease. Normal microglial responses are typically characterized by acute inflammatory phases leading to a timely resolution process; however, in AD, this response is derailed, resulting in chronic neuroinflammation that exacerbates disease progression.
Specialized pro-resolving mediators (SPMs), derived from omega-3 polyunsaturated fatty acids (n-3 PUFAs), have emerged as promising candidates for counteracting the pathological features of AD. These lipid mediators support microglial functions and potentially enhance their ability to clear amyloid-β, thus reducing neurotoxic builds-up that is commonly seen in AD pathology.
Current research has demonstrated that n-3 PUFAs make up about 20% of brain phospholipid composition. This significant percentage reflects their critical role in maintaining neuronal health and regulating inflammatory responses. Further studies indicate that SPMs can restore mitochondrial functionality in microglia, effectively boosting energy metabolism crucial for optimal immune responses. Enhanced mitochondrial respiration via SPMs has been linked to increased clearance of amyloid-β and improved synaptic integrity.
Moreover, individuals suffering from Parkinson’s disease (PD) showcase lower cerebrospinal fluid (CSF) levels of inclusion such as RvD1 and RvD2 compared to healthy controls, signaling a possible extension of research into conditions surrounding neurogenerative diseases. In animal models, specifically 5xFAD mice, treatment with SPMs like RvE1 significantly reduced both amyloid-β plaque deposition and inflammatory mediators, suggesting a beneficial influence of these mediators in neuroinflammatory settings.
As attention turns towards effective therapies, one study involving 320 cognitively unimpaired participants at increased risk of AD dementia uncovered a direct relationship between blood serum levels of alpha-linolenic acid (ALA) and eicosapentaenoic acid (EPA) with brain glucose uptake. Notably, a 2023 longitudinal analysis comprising 1,135 healthy participants before a dementia diagnosis emphasized the potential of sustained n-3 supplementation in mitigating AD incidence and cognitive decline.
The significance of n-3 PUFAs is further accentuated with findings from the DO-HEALTH trial, indicating daily intake of 1 gram could delay biological aging metrics. Enhancing dietary intake of n-3 PUFAs appears crucial not only for current AD treatment strategies but also as a preventive measure against cognitive decline.
However, clinical evidence substantiating the efficacy of SPMs in human populations remains limited. Cross-sectional studies have assorted cognitive performance correlations based on SPM levels, such as LXA4 and RvD1, with positive relationships observed with Mini-Mental State Examination (MMSE) scores among patients with varying cognitive impairments.
The underlying mechanisms by which SPMs exert their benefits largely hinge on their ability to positively influence microglial metabolism and mitochondrial function. In models of AD, the impaired mitochondrial function leads to increased ATP production deficits, reflecting inadequate energy to support homeostatic microglial functions when addressing amyloid-β presence.
Exciting advancements uncover the interplay between SPMs and sirtuin 1 (SIRT1), a pivotal metabolic regulator, confirming the role of SPMs in promoting mitochondrial health and thus influencing inflammatory responses positively. Evidence suggests that SPMs like RvD1 enhance SIRT1 expression, provide neuroprotection, and mitigate pathological progression in experimental AD cases.
Sex differences also loom large in this discourse, with women projected to have a higher lifetime risk of developing AD compared to men. This observation could be tied to hormonal influences affecting metabolic pathways in the context of aging and neurodegeneration.
Despite the promising research, substantial hurdles remain. Evaluating SPMs as therapeutic agents necessitates further investigative efforts focusing on their metabolomic profiles and clinical outcomes across diverse populations. Hence, the establishment of multifaceted biomarker panels that encompass mitochondrial function indicators holds potential promise for predicting AD trajectories and forging pathways for early intervention.
bservational studies advocating balanced n-3 dietary patterns reveal critical insights into preventive methodologies against AD. Increased consumer awareness, government campaigns promoting healthy eating habits, and structured nutritional education are pivotal to instill significant behavioral changes, aligning people towards greater n-3 consumption.
The convergence of lipidomics and neurodegenerative disease research heralds a transformative frontier in understanding AD pathophysiology. Focused research examining the implications of SPMs derived from n-3 PUFAs on mitochondrial respiration and their broader effect on microglial inflammation promises exciting ventures into innate treatment approaches.