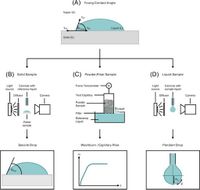
The surface free energy (SFE) of materials plays a crucial role in defining the interactions between interfaces, particularly in the realm of lithium-ion batteries (LIBs). A recent study published in Scientific Reports reveals how varying the SFE of binders used in the manufacturing of cathodes and anodes can significantly alter their properties and overall battery performance.
In the quest for more efficient and durable electric vehicle batteries, researchers have turned their attention to the interface between different battery components. Specifically, the choice of binder—namely polyvinylidene difluoride (PVDF) latex and sodium carboxymethyl cellulose (CMC)—and their respective surface free energy compositions has proven to be pivotal in optimizing the performance of lithium-ion batteries.
For cathodes, the study found that differing SFE in binders affects critical properties such as adhesion strength, electrical resistance, and water retention. These differences translate into measurable impacts on the rate capability and long-term cycling stability of the batteries. For instance, the adhesion strength of electrodes varied significantly with different binders, with uncalendered samples containing Latex 1 fully delaminating, while Latex 4 showed remarkable adhesion improvements under heat treatment.
"As the demand for high-performance batteries continues to rise, understanding these interfaces is critical to further optimize cells," the authors of the article stress, highlighting the importance of binders which, although often considered minor components, affect the mechanical integrity and electrochemical stability of the electrodes.
The researchers carefully measured the SFE of various cathode materials, revealing that common binding agents not only influence the strength of the adhesion between layers but also play a critical role in the overall electrical properties and stability of batteries. The SFE was calculated via measuring contact angles of liquid droplets on the surface of the electrodes, helping to understand the interactions between electrode materials.
Variable adhesion strengths were documented, with Latex 4 achieving an uncalendered adhesion strength of 22.5 N/m, which further increased to a remarkable 31.4 N/m after secondary drying processes. In stark contrast, the adhesion properties of Latex 1 were insufficient, resulting in poor performance and delamination.
Interestingly, Residual water content in the electrodes, which can severely affect battery performance, ranged significantly across different binders. After a short drying process of four hours at 110 °C, the residual water content for different latex systems varied from 906 ppm to 1168 ppm—highlighting how differences in binder composition can lead to substantial variations in water retention.
The findings revealed that higher polar component latexes exhibited increased water retention capabilities which can directly influence how well the solid electrolyte interphase (SEI) forms and stabilizes during battery operation. The study indicated that at 0.1 C discharge rates, Latex 2 achieved a specific discharge capacity of 127.6 mAh/g, while lower performance was seen with Latex 4 due to increased resistance attributed to higher water retention and poorer adhesion properties.
This complex interplay between binder composition, water retention, and overall electrochemical performance in lithium-ion cells is particularly important as batteries undergo charging and discharging cycles. The study demonstrates that while extensive drying processes reduce water content, they also correlate with improved electrode stability, reducing potential side reactions that can degrade performance over time.
The research provides critical insights into optimizing the manufacturing processes of lithium-ion batteries through an advanced understanding of surface free energy—a parameter that, if harnessed effectively, could pave the way for the next generation of energy storage solutions aimed at electric vehicles and renewable energy technologies.
Ultimately, the work emphasizes that improving adhesion properties and controlling water retention in electrode production systems could lead to significant advancements in the efficiency and longevity of lithium-ion batteries, solidifying their role in the energy landscape of the future.