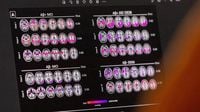
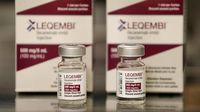
The European Commission has made a groundbreaking decision by approving the antibody drug Lecanemab for the treatment of early-stage Alzheimer's disease. Announced on April 15, 2025, this marks the first time a therapy targeting the underlying disease processes of Alzheimer’s has been authorized in the European Union.
Lecanemab is designed to address amyloid deposits in the brain, which are believed to play a significant role in the progression of Alzheimer's. While the drug aims to slow the disease's advancement, it does not offer a cure or a significant improvement in cognitive function, as experts emphasize that a complete resolution of Alzheimer's remains elusive.
According to the European Commission, the approval comes with strict conditions, as the benefits of Lecanemab outweigh the risks for a specific group of patients. This decision aligns with the recommendations made by the European Medicines Agency (EMA) back in November 2024.
Experts have noted that only a small fraction of the approximately 1.2 million Alzheimer’s patients in Germany will be eligible for this therapy. The early phase of the disease, which is critical for treatment with Lecanemab, is typically defined as the first three years after diagnosis. Currently, this early phase is estimated to affect around 250,000 individuals in Germany.
Of these, about 80 percent are eligible based on genetic factors related to the ApoE4 gene, which is associated with an increased risk of Alzheimer's. However, not all eligible patients will meet the necessary conditions or express interest in the treatment. Experts conservatively estimate that only about ten percent of those who qualify will actually receive Lecanemab, translating to roughly 20,000 patients.
The treatment is administered as an intravenous infusion every two weeks, and the costs associated with Lecanemab are significant. In the United States, the annual cost is approximately $26,500 (around €23,000) per patient, with additional upfront diagnostic expenses ranging from €1,400 to €5,000. The annual costs for administering the drug are estimated to be between €6,000 and €8,000.
Despite the promising nature of Lecanemab, experts caution against excessive optimism. The observed clinical effects of the drug have been found to be significantly lower in women than in men, raising concerns about its efficacy among the female population. Women constitute about two-thirds of all Alzheimer’s patients, and their higher risk of side effects complicates the treatment landscape.
Walter Schulz-Schaeffer, a professor at the University Hospital of Saarland, expressed skepticism regarding the practical relevance of the drug's effects, stating, "Once the full picture of Alzheimer’s disease is present, the statistically described effects are often no longer perceptible for the patient and their environment." This highlights the challenges in translating clinical trial results into real-world benefits for patients.
Furthermore, the approval process for Lecanemab requires the manufacturer to develop comprehensive guidelines and training for healthcare providers, as well as establish a monitoring registry to track patient outcomes. Experts predict that it may take several months before the drug is widely available for use in clinical settings.
In summary, while the approval of Lecanemab represents a significant milestone in the fight against Alzheimer’s disease, the practical implications for patients remain complicated. The limited eligibility criteria, potential side effects, and cost considerations create a complex landscape for both healthcare providers and patients. As researchers continue to explore new treatments, the hope for a definitive cure for Alzheimer’s persists, but for now, Lecanemab offers a modest step forward in managing this challenging condition.