In a groundbreaking study that could transform our understanding of high-entropy alloys, researchers have delved into the corrosion behavior of Al0.5Ti3Zr0.5NbxMo0.2 high-entropy alloys, discovering significant implications for high-performance materials used in harsh environments. The study, published in Nature Communications, reveals how lattice structures impact corrosion resistance and the formation of protective passivation films.
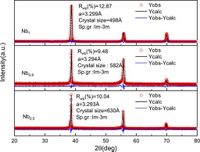
High-entropy alloys (HEAs) are a new class of materials that consist of multiple principal elements, resulting in unique structural and chemical properties compared to traditional alloys. The authors synthesized a single-phase body-centered cubic (BCC) Al0.5Ti3Zr0.5NbxMo0.2 alloy exhibiting impressive corrosion resistance, which they attribute to multiple factors enhanced by increasing niobium (Nb) content.
The research demonstrates that the structural lattice of the high-entropy alloy has a crucial effect on its corrosion behavior. Specifically, the authors found that with higher Nb concentrations, the spacing of the {011} crystalline planes decreased, which in turn improved corrosion resistance. This was evidenced by improved electrochemical stability and a greater breakdown potential in corrosive environments, particularly in a 3.5% NaCl solution commonly used for testing material durability.
“The results indicate that Al0.5Ti3Zr0.5NbxMo0.2 not only exhibits robust corrosion resistance but also has an extensive and stable passivation zone,” wrote the authors. The passive film formed on the alloy surface is crucial for mitigating corrosion in harsh environments, allowing the alloy to withstand conditions typically damaging to conventional metals.
The study highlights the unique mechanism through which Nb enhances the alloy's protective characteristics. During the corrosion process, Nb—a metal with a larger atomic radius—encourages the vertical diffusion of smaller atom species like aluminum (Al) and titanium (Ti), while facilitating the inward movement of oxygen (O). This interaction promotes the formation of stable, layered passivation films, primarily composed of high-valent oxides. Such a structure exhibits high breakdown potential and stability, crucial for maintaining integrity under corrosive conditions.
In their findings, the authors reported that the generated passivation films had a thickness of approximately 1 micrometer, with elemental analysis revealing a composition dominated by Ti and Al oxides. This layered passivation characteristic was paramount in protecting the base material from aggressive ionic environments.
Prior work had established that high-entropy alloys generally exhibit superior resistance to workforces compared to traditional alloys, yet the specific contribution of Nb had been less understood. The current research builds on earlier investigations that confirmed the greater overall corrosion resilience of refractory high-entropy alloys (RHEAs) when subjected to corrosion in chloride-infused environments.
This further opens pathways for the development and optimization of high-performing alloys tailored for extreme applications, such as in marine and nuclear environments, where corrosion resistance is vital.
Additionally, as the authors indicate, their work reinforces the importance of material design in engineering robust structural components. Developing such alloys can have broader implications for industries ranging from aerospace to energy, where reducing material failures due to corrosion can significantly improve safety and durability.
In summary, the study elucidates critical factors influencing corrosion behavior in high-entropy alloys, particularly emphasizing the significant role Nb plays in achieving enhanced corrosion resistance through innovative passivation film structures. Future studies may further investigate the applicability of these findings in real-world industrial scenarios to realize the full potential of these advanced materials.