Recent advances in water purification technology reveal the transformative potential of strained carbon nanotubes (CNTs) in facilitating highly efficient chlorination processes. This novel approach, detailed in a new study published in Nature Communications, leverages the unique properties of CNTs modified with intrinsic defect sites to significantly enhance the reactivity with chlorine molecules, ultimately yielding safer byproducts.
For decades, chlorination has been a mainstay technique in water treatment; however, the efficiency of this method has often been hindered by the formation of harmful disinfection byproducts. The study presents a breakthrough solution by employing strained CNT defect sites that enhance electronic interactions with chlorine, leading to a more favorable chemical transformation.
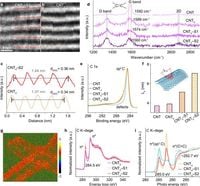
Researchers identified that these strained defect sites with elongated sp2 hybridized C–C bonds. They facilitate an initial phase of adsorption, known as Yeager-type adsorption, which governs the chlorination process. According to the authors of the article, "the reactive species in chlorination can be regulated on demand, such as the ratio of high-selectivity ClO•, ranging from 38.8% in conventional defect-based systems to 87.5% in our strain-dominated process." This increased selectivity is pivotal for generating harmless intermediates and enhancing the mineralization of pollutants.
One of the key pollutants targeted in this study was 2,4-dichlorophenol (2,4-DCP), an emerging contaminant commonly found in industrial wastewater. The application of the CNTs modified through a simple pyrolysis strategy yielded catalysts capable of dramatically escalating the degradation rate of 2,4-DCP. Specifically, the CNTd-S2 variant displayed optimal chlorine utilization and facilitated a degradation rate of 2.7 times greater than that of previous systems.
Characterization techniques including electron microscopy and spectroscopic methods were employed to confirm the presence and stability of defect sites in the CNT. The high-resolution images revealed pronounced lattice distortions in the synthesized materials, indicating successful modification and the potential for expanded catalytic activity. The findings suggest a correlation between strain fields in these catalysts and their efficiency in activating chlorine for the chlorination reactions.
Furthermore, the study demonstrated how these strained catalysts can tune the distribution of reactive species, ultimately contributing to a more effective purification process. The increase in reactive chlorine species exposure was quantified to be 13.2 times greater than existing methods based solely on CNT defects.
This advancement comes at a critical time as the demand for safe drinking water grows, underlining the urgency and importance of innovative water treatment techniques that do not compromise environmental safety. The CNTd-S2 system not only outperformed traditional methods in pollutant removal but also displayed remarkable resistance to interference from common water matrix components, including inorganic ions and organic matter.
The research team also developed a continuous-flow reactor utilizing the strained CNT membranes, achieving a remarkable degradation rate of over 99% for contaminants such as 2,4-DCP, 4-chlorophenol, and methylene blue over extended periods. This stable performance indicates a promising avenue for real-world applications in treating polluted water bodies.
With the pressing global water crisis, evolving purification methods such as this strain-dominated chlorination process hint at a future where advanced technologies can effectively combat emerging pollutants. As the study suggests, these developments not only enhance the efficiency of water treatment systems but also mitigate the potential risks associated with harmful byproduct formation.
Ultimately, the work highlights the significant role that strain engineering might play in optimizing chemical remediation technologies, paving the way for further innovations in the field of environmental science.