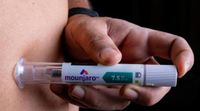
Mounjaro, a medication for type 2 diabetes developed by Eli Lilly, is set to make its debut in Brazil on June 7, 2025. This announcement was confirmed by the pharmaceutical company, detailing the drug's anticipated impact on diabetes management and weight loss.
Approved by the Brazilian Health Regulatory Agency (Anvisa) in September 2023, Mounjaro, known by its active ingredient tirzepatide, is designed for enhanced control of blood sugar. It is part of a new class of treatments that act on two incretin hormones, GLP-1 and GIP, to improve glycemic control and reduce appetite by mimicking hormones released after meals.
According to Anvisa, "Mounjaro has shown that it can provide superior glycemic control compared to other therapies available." This approval marks a significant advance in therapeutic options for diabetes, particularly as the drug also demonstrates effects beneficial for weight management, although its use for this purpose is currently considered 'off-label' in Brazil.
The maximum retail price for Mounjaro will be set at R$ 3,627.82 for a pen capable of administering six doses, a structured cost that is expected to fluctuate depending on market conditions. In the past, accessing Mounjaro required importation, costing patients between R$ 40,000 to R$ 55,000 for a six-month supply of 24 pens.
Administered via weekly subcutaneous injections, Mounjaro's unique mechanism as a dual agonist of both hormones helps to lower blood sugar levels and curb appetite more effectively than competing medications. Studies submitted by Eli Lilly illustrate that tirzepatide resulted in a body weight reduction of 47% greater than semaglutide, the active ingredient in Ozempic.
Moreover, Mounjaro is expected to be available in various dosages: 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg, allowing for personalized therapy as recommended by physicians. As Luiz Magno, Senior Director of Eli Lilly Brazil, noted, "In all markets where it was launched, it has consumption above what we are able to produce. That delayed the launch in Brazil," emphasizing the high demand that has influenced rollout schedules.
Clinical trials indicate that patients using tirzepatide, when combined with lifestyle changes such as diet modifications, saw a weight loss of up to 20%, with some studies reporting as much as 22.8 kg lost compared to only 15 kg lost by those using semaglutide.
Despite its promises, Mounjaro is not without potential side effects. According to Eli Lilly, users may experience nausea, vomiting, and diarrhea, typically of mild to moderate intensity. Caution is advised, as with all medications, necessitating ongoing monitoring by healthcare professionals.
With Mounjaro poised to enter Brazil's diabetes treatment landscape, the impact it could have on patient care is significant. Not only does it offer a new mechanism for controlling blood sugar, but it also opens doors for individuals seeking effective weight loss solutions.
While Mounjaro has been approved for diabetes management, the Anvisa is still considering its approval for obesity treatment, mirroring approaches taken in the United States where the medication is already utilized for both indications. This consideration underlines a growing recognition of the intersection between obesity and diabetes management.
The launch of Mounjaro in Brazil promises to be a pivotal moment in diabetes treatment, not only providing better glycemic control for patients but also addressing the pressing health challenge of obesity in the country.
In summary, as Mounjaro approaches its launch date, it represents a beacon of hope for many in Brazil suffering from diabetes and weight management issues, promising more effective treatment options in a field that values innovation and efficacy.