A recent study has revealed that PSMD2, a protein associated with the ubiquitin-proteasome pathway, is significantly overexpressed in hepatocellular carcinoma (HCC), a leading cause of cancer-related deaths worldwide. This increase in PSMD2 levels correlates with poor prognosis and suggests its role in the immune evasion of tumor cells, pointing to its potential as a crucial biomarker and therapeutic target in HCC.
HCC accounts for approximately 90% of primary liver cancers, and the prognosis remains dire, particularly in regions like China, where over 70% of cases are diagnosed at advanced stages. Traditional treatment options, which may succeed at early stages, offer minimal hope for these patients, underscoring the urgent need for further research into the mechanisms driving tumor progression. Immune checkpoint inhibitors (ICIs), although promising in various cancers, have shown suboptimal efficacy in HCC, with only about 10-35% of patients benefiting from such treatments.
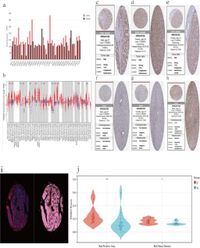
The research team employed bioinformatics methods, utilizing the GEPIA and TIMER databases to analyze PSMD2 expression levels in HCC tissues compared to adjacent normal liver tissues. Through extensive analyses, they found that high PSMD2 expression was significantly associated with worse overall survival (OS) and progression-free survival (PFS) among HCC patients, with a hazard ratio (HR) of 1.61 and a P-value of 2.0e-4.
Moreover, the study explored the relationship between PSMD2 and various immune checkpoint genes, including PD-1, PD-L1, and CTLA-4. Findings reveal a positive correlation between PSMD2 levels and the expression of these critical immune regulators, indicating that PSMD2 may facilitate tumor immune evasion through these mechanisms.
The overall survival analysis, supported by the Kaplan-Meier method, showcased that patients with elevated expression of PSMD2 had significantly shorter survival times. The area under the ROC curve further demonstrated the predictive accuracy of PSMD2 for determining long-term outcomes in HCC, reinforcing its potential clinical relevance.
In addition to survival analysis, the researchers investigated the immunological aspect by assessing immune cell infiltration. They noted that higher PSMD2 levels were negatively correlated with the presence of CD8+ T cells and the infiltration levels of several antitumor immune cells. In contrast, there was a positive correlation with macrophages and regulatory T cells (Tregs), which are known to suppress immune responses and contribute to tumorigenesis.
The study used tools such as the Tumor Immune Dysfunction and Exclusion (TIDE) analysis to elucidate the immune landscape associated with PSMD2 expression. Results indicated that patients with high PSMD2 expression had increased immune evasion potential, which aligns with the observed correlation between PSMD2 and immune checkpoint genes.
Moreover, the protein-protein interaction (PPI) network analysis conducted via the STRING database highlighted PSMD2’s interaction with key proteins involved in cancer progression and immune modulation. Notably, the enrichment analysis pointed to significant pathways in which PSMD2 is implicated, such as p53 signaling and the ubiquitin-mediated proteolysis pathway.
As the study concluded, elevated PSMD2 levels not only indicate a poor prognosis for HCC patients but may also represent a novel target for immunotherapeutic strategies, aiming to enhance the efficacy of currently available treatments by overcoming immune evasion. This research offers a significant addition to the understanding of the tumor microenvironment and highlights the importance of targeting PSMD2 to potentially improve clinical outcomes in HCC.
Future investigations are warranted to explore the specific mechanisms through which PSMD2 influences the immune microenvironment and its interaction with other cancer-associated proteins to validate its utility as a therapeutic target. In light of these findings, further research could significantly bolster treatment options for patients grappling with this challenging malignancy.