Researchers have discovered how proline isomerization and disulfide bonding regulate the dynamics of the titin Ig1 domain, a critical protein in muscle function. Their findings reveal that titin Ig1 can exist in two mechanically distinct states influenced by these interactions, with significant implications for our understanding of muscle elasticity and function.
Titin is an essential protein contributing to the mechanical properties of cardiac and skeletal muscles. It stretches from the Z-disk to the M-line in sarcomeres, the functional units of muscle fibers. The protein acts like a spring, providing elasticity and playing a vital role during muscle contraction and relaxation cycles. Importantly, titin's I-band region consists of multiple Ig-like domains, with each domain incorporating unique structural features.
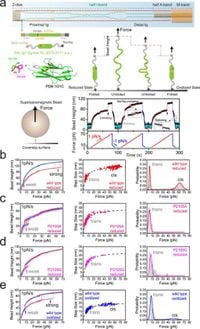
The recent study, conducted by a team of scientists from various institutions, employed single-molecule force spectroscopy to probe the mechanical behaviors of the titin Ig1 domain under varying conditions. They focused on how proline isomerization (the ability of proline to switch between cis and trans forms) and the formation of disulfide bonds ( that stabilize certain structures under oxidative conditions) influence the mechanical properties of these domains.
Evidence has shown that the titin Ig1 domain can exist in two states—cis and trans—each associated with different mechanical stability. The study found that the trans-Ig1, in a state of isomerization, is over 50 pN weaker than the cis-Ig1, unfolding approximately 1000 times faster under physiological conditions. This discovery highlights the crucial role of proline isomerization in tuning the mechanical response of titin amid dynamic physiological forces.
The presence of cysteine residues in the titin Ig domains opens a pathway for disulfide bonding, which appears to further modulate the stability of these states. Observations revealed that while both cis- and trans-Ig1 domains made use of the catch-slip bond behavior, the introduction of disulfide bonds in oxidized domains led to distinct slip-catch-slip unfolding dynamics. This presents the possibility of hidden intermediate states that may operate under various force levels, impacting how muscle tissues respond to different stresses.
This intricate behavior of the titin Ig domains demonstrates a finely-tuned regulatory mechanism, underscoring the implications of these findings for understanding muscle diseases as well as broader physiological processes where titin's functions are crucial. Additionally, with over 60% of I-band domains containing a proline in the cis-state, exploring these features further could illuminate how structural dynamics influence protein functionality.
Future research directions suggested by this study aim to investigate the broader implications of proline isomerization and disulfide bonding in different forms of titin and other related proteins involved in muscle function and mechanosensitization.